J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(3):334-344, 2003
Influence of storage conditions and type of plasticizers on ethylcellulose and acrylate films formed from aqueous dispersions.
Paul W.S. Heng1, L.W. Chan, K.T. Ong
Department of Pharmacy, Faculty of Science, National University of Singapore, SingaporeReceived 19 August 2003, Revised 11 October 2003, Accepted 3 November 2003
PDF version
Abstract
PURPOSE: To investigate the influence of storage conditions and types of plasticizers on the properties and stability of ethylcellulose and polymethacrylate films and to elucidate the mechanism for the changes observed. METHODS. Films were prepared from Surelease, Aquacoat and Eudragit L 30D dispersions by the casting method. The effects of different plasticizers on the morphology, transparency, mechanical property and water vapour permeability of the prepared films were studied. The film samples were exposed to storage conditions of 30°C and 50 or 75 %RH. Samples were removed at pre-determined time intervals for mechanical testing and analysis of plasticizer content in the films. RESULTS. It was found that films prepared from aqueous ethylcellulose dispersions were relatively weaker and more brittle than acrylate films. Acrylate films did not show any significant change in mechanical property when stored at high humidity. However, the properties of ethylcellulose films stored at high humidity varied depending on the type of plasticizers present. CONCLUSIONS. The changes in mechanical property of ethylcellulose films on storage were mainly attributed to the loss of plasticizers during storage, causing further coalescence of ethylcellulose films and to a smaller extent, reduction in moisture content of the film.
Introduction
Introduction
Many polymeric film coats applied onto a dosage form have been reported to undergo changes in mechanical properties upon storage. The extent of these changes is influenced by several factors such as the amount of plasticizers added, type of plasticizers used, film forming conditions, film storage temperature and humidity. It was found that films formed from aqueous ethylcellulose dispersions showed significant changes in their mechanical properties when they were dried at a higher temperature (1). Exposure to a high relative humidity may cause a polymeric film coat to absorb moisture from the atmosphere. Findings of several researchers suggested that changes in mechanical properties of some polymeric films stored at high humidities are correlated with the plasticizing properties of the moisture present in films. Changes in the mechanical properties of unplasticized hydroxypropylmethylcellulose films exposed to high humidity were found to be consistent with the plasticizing effect of water (2). In another study, it was reported that higher storage relative humidity resulted in a decrease in both tensile strength and Young's modulus of beads coated with Eudragit RS 30D/RL 30D containing methylparaben as a non-traditional plasticizer (3). Changes in film mechanical properties may ultimately influence the drug release, stability and the final physicochemical properties of the coated dosage forms (4).
Ethylcellulose (5, 6) and acrylates (7) are among the most commonly used polymers in the production of coated controlled-release dosage forms. As these polymers are insoluble in water, they have to be applied either in the form of an organic solution or an aqueous dispersion (8). As organic solvents are associated with many problems such flammability, explosion hazards, toxicity and environmental contamination, the use of water-based coating systems has become more popular. Under normal coating conditions, formation of continuous films from most of the polymers formulated as an aqueous dispersion is generally not possible without the addition of plasticizers. A plasticizer is a substantially non-volatile, high-boiling and nonseparating substance that changes certain physical and mechanical properties of the polymer to be plasticized (9). In particular, plasticizers are required for polymer dispersions that have minimum film formation temperatures above the coating temperature employed. During plasticization of aqueous dispersions, the plasticizer will partition into and soften the colloidal polymeric particles, thus promoting particle deformation and coalescence into a homogeneous film (10). Hence, increase in concentration of plasticizer generally decreases the softening temperature, glass transition temperature and tensile strength of the film formed. The degree of plasticization of a polymer is dependent on the amount of plasticizer in the film and interaction between plasticizer and polymer.
Two better known commercial aqueous ethylcellulose dispersions are Surelease (Colorcon Inc., US) and Aquacoat (FMC Corp., US). Surelease, which has medium chain triglycerides as a plasticizer, is employed directly for coating. Aquacoat, which does not contain any plasticizer, has to be externally plasticized before use. Several researchers have studied the effects of different types of plasticizers on the mechanical properties of Aquacoat films (1, 7). Plasticizers such as dibutyl sebacate, tributyl citrate, acetyl tributyl citrate and oleyl alcohol were found to produce ethylcellulose films that showed greater elongation upon stretching, after the films had been stored under conditions of elevated humidity (1). However, the actual mechanisms that caused the above change and the extent of influence by the different types of plasticizers have not been reported.
The primary objective of the present study was to compare the influence of storage humidity and temperature on the mechanical properties and water vapour permeability of ethylcellulose and polymethacrylate films. This study also investigated the effects of different types of plasticizers on the properties and stability of the films exposed to different storage conditions and attempted to elucidate the mechanism for the changes observed.
MATERIALS AND METHODS
Materials
Aqueous dispersions of the following film formers were used: methacrylic acid-ethyl acrylate copolymer (MA; Eudragit® L 30D, Röhm Pharma, Darmstadt, Germany) and ethylcellulose (EC; Aquacoat®, type ECD-30, FMC Corp., Newark, US and Surelease®, Colorcon, West Point, US). The plasticizers used were dibutyl phthalate (DBP), triethyl citrate (TEC), glycerin triacetate (GTA) from Merck-Schuchardt (Darmstadt, Germany) and diethyl phthalate (DEP) from The British Drug House (Poole, UK). The plasticizers, acetyltriethyl citrate (ATEC) and acetyltributyl citrate (ATBC) were donated by Morflex (Greensboro, US).
Preparation of Film Forming Dispersions
Three types of aqueous polymeric dispersions studied were Surelease, Aquacoat and Eudragit L 30D. Except for Surelease which already contained medium chain triglycerides as a plasticizer, 30 %w/w (based on polymer weight) plasticizers were added to the polymeric dispersions which were then diluted with water to 10 %w/w. The plasticizers were used at level suggested by the manufacturer. The water-soluble plasticizers (TEC and GTA) were stirred in the polymeric dispersions for 5 h using magnetic stirrers while the water-insoluble plasticizers (DEP, DBP, ATEC and ATBC) were stirred for 24 h.
Preparation of Free Films
The free films of about 200 m m thick (dried) were prepared by casting pre-determined amounts of polymeric dispersion on levelled polytetrafluoroethylene (PTFE)-coated glass plates (casting area = 17 cm × 17 cm). The dispersions were carefully handled to prevent the formation of air bubbles. Films from Eudragit L 30 D were dried at 40°C and the rest at 55°C.
Evaluation of film properties
The free films were cut into strips of 70 mm × 10 mm for evaluation of mechanical properties or circular pieces with diameter of 7.46 cm for determination of water vapour permeability. Film thickness was determined by measuring the thickness at five scattered points on the film, using a digital micrometer (Mitutoyo, Japan) and the values averaged. Only film samples with mean thickness within the range of 180 to 220 mm and thickness value variation less than 10 % in each film were used for the above tests.
Film samples used for mechanical testing, assay of plasticizer and moisture content and determination of percent weight change were stored in controlled environment chambers of 30°C and 50 or 75 % RH. Samples were removed after 0, 3, 7, 14, 28 days of storage for evaluation. For mechanical testing, the film samples were equilibrated at ambient room condition of 22 ± 2°C and 55 ± 2 % RH for 1 hour prior to testing. This is to minimise any variations in result due to room condition.
Mechanical Properties
The mechanical properties of the films were evaluated using a tensile testing instrument (EZ Test-100N, Shimadzu, Japan) mounted with a 100 N capacity load cell. The test procedure was based on the ASTM D 882 - 75d (11) method using flat-faced metal grips with surfaces laminated with sand paper for better hold. The initial gauge length was set at 50 mm and the extension speed was 5 mm/min. The tests were carried out at ambient conditions of 22 ± 2°C and 55 ± 2 %RH.
Four mechanical properties, namely tensile strength, % elongation at break, elastic modulus and work of failure were computed from the load - strain profile, and film dimensions as shown below.
t = Lmax/Ai (1)
e= ∆lb/li (2)
EM =dL/dm/Ai ( 3)
w = AUC x δ/Ai (4)
where, t is the tensile strength, Lmax, the maximum load, Ai, the initial cross-sectional area of the sample, e, the percent elongation at break, ∆lb, the increase in length at break point, li, the initial gauge length, EM, the elastic modulus, dL/dm, the slope of the linear potion of the elastic deformation, w, the work of failure, AUC, the area under the curve and δ, the cross-head speed. At least five measurements were taken and the average calculated for each film formulation.
Plasticizer content
The amount of plasticizer in the Aquacoat film was determined using gas chromatography (Model 5890 series II Hewlett Packard) with a split/splitless inlet, a 23.5 m by 0.32 mm fused silica-polyethylene glycol capillary column (HP-FFAP X-linked polyethylene glycol) and a flame ionization detector. About 200 mg of film sample were accurately weighed and dissolved in methanol. An internal standard of 1 ml was then added to the mixture. TEC (5 mM) was employed as the internal standard for GTA while GTA (10 mM) solution was the internal standard for the rest of the plasticizers. Using methanol, the mixture was made up to a final volume of 20 ml for DBP and 10 ml for the rest. Nitrogen was used as the carrier gas at a flow rate of 28.5 ml/min, with injector temperature at 240°C and detector temperature at 270°C. For the assay of DEP, TEC, ATEC and GTA, the column temperature was increased from 150°C to 230°C at a rate of 10°C/min and held at 230°C for 3 min. For the assay of DBP and ATBC, the column was heated up from 150°C to 240°C at a rate of 10°C/min and held at 240°C for 5 min.
Percent weight change of film
Free film samples of 2.5 cm by 4.5 cm were accurately weighed and stored in the controlled environment chambers at 30°C and 50 or 75 %RH. At specified time intervals, the films were removed and weighed immediately. The percent change in weight was computed from the difference between the final and initial weights, with respect to the initial weight of the film.
Moisture content
The moisture content of the film was determined by Karl Fischer analysis (701 KF Titrino, Metrohm, Switzerland). About 0.2 g of film samples, accurately weighed, was first dissolved in methanol before titration. At least 4 sets of measurements were obtained for each film formulation. The moisture content of film was expressed as percent weight of film moisture with respect to weight of film. The percent change in moisture was represented by the difference between the final and initial moisture content, with respect to the initial moisture content of the film.
Water Vapour Permeability
Water vapour permeability of the films was determined using the ASTM water vapour transmission test method (Dry cup method, ASTM E 96 - 95) (12). The film samples were conditioned by storing at ambient condition of 22 ± 2°C and 55 ± 2 %RH for at least 5 days. The drying agent, silica gel beads, was activated by heating at 200°C. A sample of 15 g of the silica gel beads was placed in each aluminium permeability cup (Paul Garner, US) and then sealed with film sample sandwiched between rubber and PTFE gaskets. The cup was then tightly closed with a screw cap with an opening exposing an effective film area of 24.5 cm2 for water vapour permeation. The whole assembly was weighed and placed in a controlled environment chamber set at 30°C, 50 % RH or 30°C, 75 % RH for 30 h. At specified time intervals, the cup was briefly removed and weighed. At least 3 sets of measurements were obtained for each film formulation. Plots of weight gain against time were constructed.
The water permeation rate was calculated using the following formula,
Rwvp = W/ (A x ∆ p) (5)
where Rwvp , is the water vapour permeation rate (g h-1 cm-2 mmHg-1 ), W, the amount of water vapour permeated through the film (g h-1 ), A, the area of exposed film (cm2 ), and ∆p, the vapour pressure difference (mmHg).
Assuming that the air on the desiccant side was dry (i.e. 0 mmHg of water), the water vapour pressure difference across the film at 30°C was 15.5 mmHg and 23.8 mmHg for cups stored at 50 % RH and 75 % RH respectively (13). The permeability, P was calculated as follows:
P = R wvp x t (6)
where t is the thickness of the film.
Surface Morphology
Surface morphology of the film was examined using a scanning probe microscope (SPM-9500J, Shimadzu, Japan). The film samples were scanned over an area of 25 mm by 25 mm.
Film Transparency
Strips of film (2.5 cm × 4 cm) were equilibrated at ambient condition of 22 ± 2°C and 55 ± 2 %RH for 5 hours. Each strip was mounted on the cell holder of a spectrophotometer (UV-3101 PC, Shimadzu, Japan) and light transmittance at 600 nm was determined. Three strips of films were used for each film formulation and the mean percent transmittance calculated.
RESULTS And Discussions
Morphology of the films
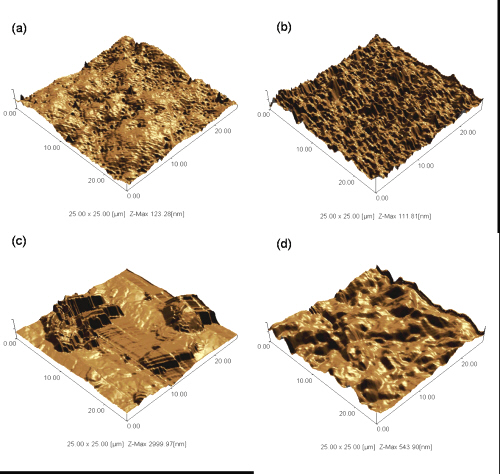
Both Eudragit L 30D and Surelease films appeared homogeneous and continuous. The texture of Aquacoat films varied with the type of plasticizers used. Aquacoat films plasticized with DBP appeared to be more flexible, smoother and homogeneous while those plasticized with DEP and ATEC had raised spots and undulating surfaces. Orange peel appearance was also observed for some of the Aquacoat films. SPM images showed that Eudragit L 30D and Surelease films were relatively smooth with small perturbations and peak heights of less than 0.5 mm. In contrast, Aquacoat films had rougher surfaces with prominent protrusions (Figure 1).
Figure 1: SPM images of MA (a), Surelease (b), and Aquacoat films plasticized with DEP (c) and DBP (d).
The average peak heights for the plasticized Aquacoat films were greater than 0.5 mm, with DEP plasticized Aquacoat films having the highest mean peak height of more than 2 mm.
All the freshly dried Eudragit L 30D, Surelease and Aquacoat films, except those plasticized with ATEC, appeared transparent initially. Upon exposure to ambient condition of 22 ± 2°C and 55 ± 2 %RH for about 5 h, Eudragit L 30D and Surelease films showed little change in transparency but Aquacoat films became translucent as shown by their markedly lower transmittance (Table 1).
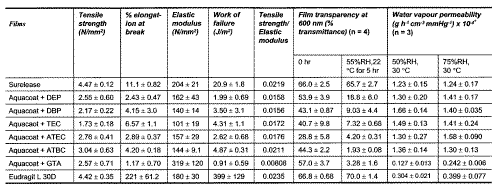
Table 1: Comparison of mechanical propertiesa, film transparency and water vapour permeabilityb of equilibrated freshly prepared EC and MA films. (n = 5 unless otherwise specified).
*Films were equilibrated at 55 ± 2 %RH and 22 ± 2°C for a1 hour b5 days before test.
Similar findings for Aquacoat films had been reported (1). Emulsifying agents are required to form stable and finely divided aqueous dispersion of the practically insoluble ethylcellulose. It was suggested that the translucency of Aquacoat films might be due to the migration of the emulsifying agents to the minuscule `islets' of the film (14, 15).
Mechanical properties
An ideal film coat should be hard and tough without being brittle. These properties can be defined in terms of elastic modulus, tensile strength, % elongation at break (16) as well as work of failure (17). Elastic modulus is a key indicator of stiffness or rigidity of polymer films (3), while % elongation at break can be used to predict the ductility of the film. Hence, a hard and tough polymer film has high elastic modulus, high tensile strength and high % elongation at break while a soft but tough polymer film has low elastic modulus, moderate tensile strength and high % elongation at break. Tough films are also characterized by high work of failure. The ratio of tensile strength to elastic modulus could be related to in situ performance of the film (18). It is desirable to have films with higher ratio of tensile strength to elastic modulus as coating defects were observed to increase with lower values of this ratio.
The mechanical properties of the films are summarized in Table 1. Eudragit L 30D plasticized with TEC formed a tough film as shown by the high % elongation at break and work of failure values, at least 19 times higher compared with the ethylcellulose films. Surelease and Aquacoat films had relatively low % elongation at break and work of failure values, indicating that they were brittle. Bodmeier et al. (7) suggested that the interchain hydrogen bonding and bulkiness of glucose subunits of the polymer might have given rise to the brittle nature of the film. Compared to Aquacoat films, Surelease films were tougher as they had higher work of failure, % elongation at break values, as well as higher tensile strength to elastic modulus ratio. It was suggested that the presence of sodium lauryl sulfate in Aquacoat might have been responsible for the lower tensile strength (7). The higher % elongation at break also showed that Surelease films were better plasticized. Surelease is stabilized by an anionic surfactant, ammonium oleate in combination with a plasticizer composed of medium chain triglycerides (19). During film drying, the less stable ammonium oleate would be converted to oleic acid that could act as an additional plasticizer for ethylcellulose, resulting in a better plasticized film.
Despite varying markedly in the values of % elongation at break and work of failure, the films prepared from Surelease, Eudragit L 30D plasticized with TEC and Aquacoat plasticized with ATBC showed comparable ratios of tensile strength to elastic modulus (0.0211 to 0.025). Compared to the rest, these 3 films had the highest ratio value and were expected to produce the least coating defects. Marked variation in the ratio values (0.00808 to 0.0211) was observed for Aquacoat containing different types of plasticiziers, indicating the important effects of plasticizers.
Influence of plasticizers on properties of films
The effects of plasticizers on mechanical properties of ethylcellulose films were also compared. The plasticizers used were classified into 3 types: phthalic acid esters (DEP, DBP), citric acid esters (TEC, ATEC, ATBC) and glycerol acid ester (GTA). DBP and ATBC increased the % elongation at break and work of failure of the films to a greater extent than the corresponding DEP and ATEC (Table 1), suggesting that the plasticizers with larger alkyl substituents produced tougher EC films. This phenomenon was probably associated with higher hydrophobicity of the plasticizers, which was more compatible with water-insoluble ethylcellulose. It should be recalled that DBP-plasticized films were smoother and homogenous while DEP- and ATEC-plasticized films had raised spots and undulating surfaces. No clear trends between the effects of the critic acid esters and phthalic acid esters on the % elongation at break and work of failure of the films were observed. However, the critic acid esters were found to produce films with relatively higher tensile strength to elastic modulus ratios than phthalate acid esters. The glycerol ester, GTA, produced films with the lowest ratio value. The influence of the class of plasticizers on the ratio value was noted. Ethylcelluose consists of ether and hydroxyl groups which are capable of interacting with plasticizer molecules via hydrogen bonds. The 3 classes of plasticizers have different molecular structures and number of functional groups for hydrogen bonding and are expected to show different extents of interaction with ethylcellulose. Hence the extent of interaction between the plasticizer and the polymer has an important influence on the tensile strength to elastic modulus ratio of the film produced. In this respect, phthalic acid esters are generally better plasticizers than citric acid and glycerol esters.
Influence of storage time on properties of films
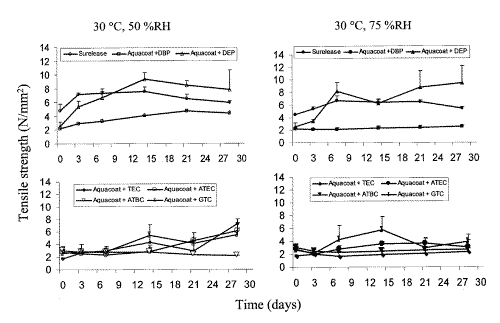
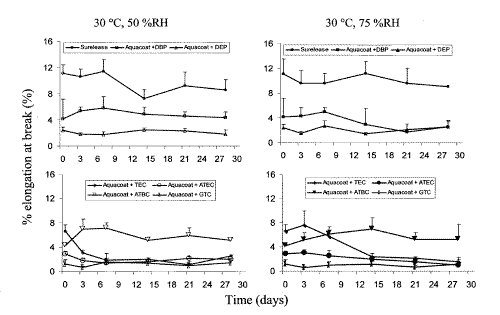
Mechanical properties of a film are critical as they affect the intended function of the film in a dosage form. These properties were found to vary to different extents with time when the plasticized EC films were stored at 30°C, 50 %RH for over 4 weeks. (Figures 2-5).
Figure 2: Effect of storage conditions on tensile strength of ethylcellulose films: Surelease and Aquacoat films plasticized with DBP, DEP, TEC, ATEC, ATBC, GTA stored at 30°C, 50 %RH and 30°C, 75 %RH (n=5, Mean + SD).
Figure 3: Effect of storage conditions on % elongation at break of ethylcellulose films: Surelease and Aquacoat films plasticized with DBP, DEP, TEC, ATEC, ATBC, GTA stored at 30°C, 50 %RH and 30°C, 75 %RH (n=5, Mean + SD).
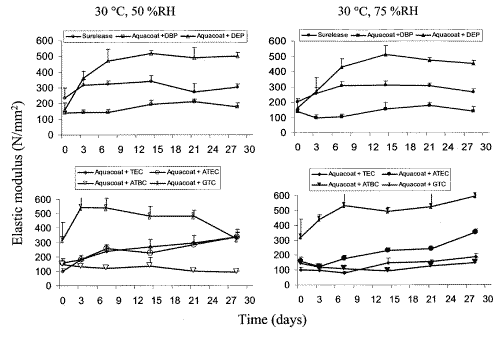
Figure 4: Effect of storage conditions on elastic modulus of ethylcellulose films: Surelease and Aquacoat films plasticized with DBP, DEP, TEC, ATEC, ATBC, GTA stored at 30°C, 50 %RH and 30°C, 75 %RH (n=5, Mean + SD).
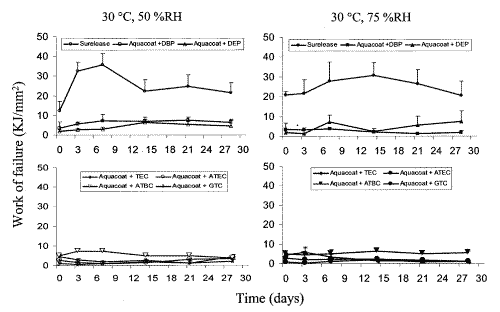
Figure 5: Effect of storage conditions on work of failure of ethylcellulose films: Surelease and Aquacoat films plasticized with DBP, DEP, TEC, ATEC, ATBC, GTA stored at 30°C, 50 %RH and 30°C, 75 %RH (n=5, Mean + SD).
The ATBC- and DBP-plasticized films were the most stable as they showed little change in the four mechanical properties studied while TEC- and DEP-plasticized films were the most unstable. With the exception of Surelease films, the tensile strength and elastic modulus of the plasticized films were generally more affected than their % elongation at break and work of failure. Increase in tensile strength and decrease in elastic modulus were observed in most cases, indicating that the films were gradually becoming brittle and rigid on storage.
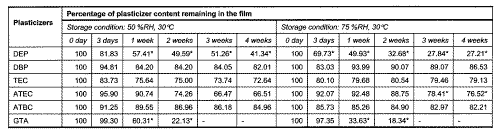
Assays of DEP, DBP, GTA, TEC, ATEC and ATBC remaining in Aquacoat films at different time intervals were carried out. The amounts of DEP and GTA were found to decrease significantly after a week of storage at 50 %RH (Table 2).
Table 2: Comparison of plasticizer content in Aquacoat films exposed to storage conditions of 50 %RH, 30°C and 75 %RH, 30°C. (n = 5).
*values significant at p<0.05 compared to initial content in film before storage.
The amount of GTA was not detectable after 3 weeks of storage. More than 30 % of ATEC was also lost during storage at 50 %RH though it was not statistically significant. The loss of plasticizers could be attributed to volatization or degradation of the plasticizers during storage. The percent weight changes of films stored at 50 %RH and 75 %RH were also determined (Table 3).
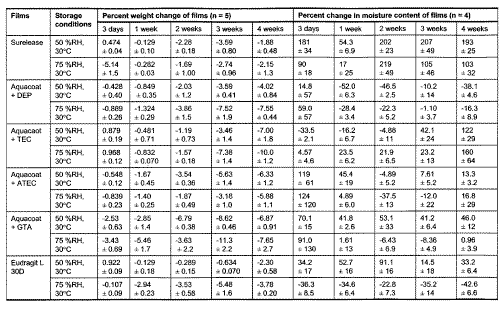
Table 3: Comparison of percent weight change and percent change in moisture content of films exposed to storage conditions of 50 %RH, 30°C and 75 %RH, 30°C.
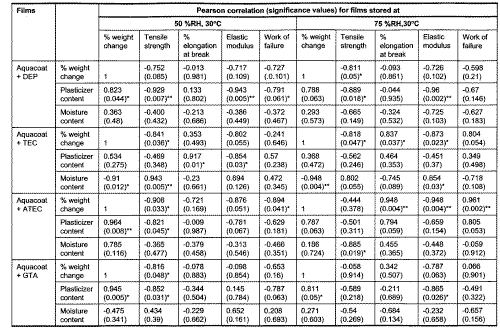
All Aquacoat films, except for those plasticized with ATBC, generally showed decline in weight during storage. The percent weight loss was greatest for Aquacoat plasticized with GTA, ATEC, TEC and DEP. A two tailed correlation analysis was conducted for percent weight change, plasticizer content, moisture content and mechanical parameters of Aquacoat films stored at 50 %RH. Only Pearson correlations for percent weight change and plasticizer content of Aquacoat films plasticized with DEP, GTA or ATEC were found to be statistically significant at 0.05 levels (Table 4).
Table 4: Pearson correlation for films exposed to storage conditions of 50 %RH, 30°C and 75 %RH, 30°C.
*values significant at p<0.05
**values significant at p<0.01
This suggested that the decline in percent weight change of Aquacoat films plasticized with DEP or GTA stored for more than 3 days was primary due to loss of plasticizers. Other researchers have also reported loss of plasticizer, such as propylene glycol, during storage (20, 21). Statistical analysis also showed significant correlations between tensile strength or elastic modulus and DEP content of films stored at 50 %RH. Pearson correlation between tensile strength and GTA content of films was also found to be significant at 0.05 levels. This showed that the changes in mechanical properties observed were largely due to loss of DEP or GTA from films. Similar findings were reported in the study of Eudrgait L 30D films plasticized with triacetin (22).
Changes in mechanical properties of Aquacoat films plasticized with TEC and ATEC also indicated a hardening of film resulting in increased brittleness. The determination of TEC content by gas chromatography in Aquacoat films showed a loss of approximately 25% after a week of storage at 50 %RH. However, no further loss in TEC content was observed on prolonged storage. By contrast the elastic modulus and % elongation at break values continued to show changes beyond a week of storage. Table 4 shows a significant (p<0.05) correlation between TEC content and % elongation at break or elastic modulus of films. This suggests that the initial changes of Aquacoat films were due to TEC loss. After prolonged storage, other factors had contributed to the continual hardening of the films.
The mechanism for film formation of latex systems has been proposed by many researchers (23, 24, 25). Each submicron-size polymer consists of hundreds of polymer chains. The film formation involves coalescence of these polymer particles into a homogeneous continuous film as the aqueous phase evaporates. As water evaporates, interfacial tension between water vapour and polymer pushes the particles into close packing as ordered arrays. A strong driving force, exerted mainly by capillary action between particles, is necessary to overcome repulsive forces, deform the particles and cause them to fuse. The film forming process depends on the manufacturing method, coating conditions and formulation variables. It was also reported that the degree of coalescence of latex particles increased with storage time (26). In most of the studies, the degree of coalescence was found to be greatly affected by storage temperature (1, 26, 27). It was likely that the changes in mechanical properties and loss of weight of Aquacoat films plasticized with TEC and ATEC on prolonged storage was mainly due to the phenomenon of coalescence. On the other hand, changes in mechanical properties of Aquacoat films plasticized with DEP and GTA were probably influenced by two factors, loss of plasticizers and increasing coalescence of polymer particles during storage. This explained the greater magnitude of change in tensile strength and elastic modulus, as well as percent weight change observed for Aquacoat films plasticized with DEP and GTA.
Influence of storage humidity on properties of films
The mechanical properties of ethylcellulose films stored at medium and high humidities of 50 %RH and 75 %RH was compared and shown in Figures 2-5. A humidity of 75 % represents a high humidity associated with outdoor conditions whilst 50 %RH is medium level, reflecting indoor controlled environment conditions commonly seen in pharmaceutical manufacturing facilities. Similar trends were generally observed but the magnitude and rate of change were different. Changes in the mechanical properties were not always greater for films stored under higher RH of 75 %. For example, the tensile strength of DBP-plasticized films stored at 50 %RH increased significantly with time while those stored at 75 %RH had relatively constant tensile strength. The magnitudes of change in tensile strength and elastic modulus of Aquacoat plasticized with DEP after 3 days of storage at 50 %RH were also greater compared to those stored at 75 %RH for the same number of days. Aquacoat films plasticized with citrate ester plasticizers, TEC and ATEC, also showed similar differences in magnitude of change in tensile strength, elastic modulus, as well as, % elongation at break when stored at different humidities. These differences in magnitude of change suggest that storage at higher humidity also affected the changes in mechanical properties of plasticized Aquacoat films to a significant extent.
The moisture contents of the films stored at 50 %RH and 75 %RH were determined (Table 3). Most of the plasticized Aquacoat films stored at both humidities have showed an initial increase in moisture content, ranging from 6 % to 123 % after 3 days of storage. Except for Aquacoat films plasticized with TEC and GTA, all the Aquacoat films showed similar decline in moisture content on prolonged storage to lower than or almost similar to initial values. However, there was no significant correlation between the moisture content and mechanical parameters of the Aquacoat films, except those plasticized with TEC (Table 4). The smaller magnitude or delayed trend of change in mechanical properties of some Aquacoat films suggested that a greater absorption of moisture might have occurred for films stored at higher humidities, though the assay did not show a higher moisture content for films stored at 75 %RH compared to those stored at 50 %RH. However, the influence of moisture on Aquacoat films is temporal and on prolonged storage, other prominent factors such as loss of plasticizers and further coalescence resulted in a greater change in film structure.
For example, Aquacoat films plasticized with ATBC, another citrate plasticizer, did not show significant change in the mechanical properties except a small rise in elastic modulus values. This slight increase might be due to the small amount of ATBC loss from Aquacoat films during storage. It is interesting to note that the moisture content of Aquacoat plasticized with ATBC showed the greatest increase after 1 week, but decreased markedly to near initial value after 2 weeks of storage at both humidities. The percent weight change result also reflected a small but sustained increase in weight of the film though this increase was found not to be significant. The variation in moisture content however did not affect the mechanical properties of Aquacoat films plasticized with ATBC stored at a high humidity. This suggested that Aquacoat plasticized with ATBC was less influenced by moisture in the environment than those plasticized by other citrate plasticizers.
Films containing GTA which is soluble in water, showed a marked increase in moisture content when stored at both 50 %RH and 75 %RH (Table 3). However, while the moisture content of Aquacoat film plasticized with GTA stored at 50 %RH remained almost constant after 1 week of storage, those stored at 75 %RH showed a significant subsequent decline to near initial value on prolonged storage. This may be due to the greater drop in GTA content to less than 20 % after 2 weeks of storage of the films at 75 %RH. The accelerated decline in GTA content of Aquacoat films stored at 75 %RH therefore resulted in lower tendency for Aquacoat film to absorb moisture from the environment. The high loss in GTA content of Aquacoat films stored at both humidities caused modifications in film structure resulting in increased rigidity and hence explained the similar change in mechanical properties observed for the films stored at both humidities.
Compared to Aquacoat films, the differences in mechanical properties of Surelease films stored at both 50 %RH and 75 %RH were less marked. A small but significant (p<0.05) increase in tensile strength was observed for Surelease films stored at both 50 %RH and 75 %RH. Independent sample T-tests were conducted and the differences between the tensile strength values for Surelease stored at 50 %RH and 75 %RH for 1, 2 and 4 weeks, were found to be statistically significant at 95 % confidence interval. The percent weight change of Surelease films showed a small decline over 4 weeks of storage at both 50 %RH and 75 %RH (Table 3). On the contrary, the moisture content showed a significant increase after 3 days of storage. This indicated that other more prominent factors, such as greater coalescence, were responsible for the weight loss of Surelease films. Unlike ethylcellulose films, there was no prominent change in mechanical properties of MA films stored at high humidities for up to 4 weeks. A small but significant decrease in film weight was observed after 4 weeks of storage (Table 3). This may be due to small loss in moisture for films during storage at 75 %RH.
Water vapour permeability
The water vapour permeabilities of EC and MA films are shown in Table 1. With the exception of Aquacoat film plasticized with GTA, the rest of the EC films were at least thrice more permeable to water vapour than the plasticized Eudragit film and only slightly more permeable than Surelease films. This implies that the plasticized MA films were denser in structure compared to the plasticized EC films. There was no significant difference in the permeabilities of EC films plasticized with DBP, DEP, TEC, ATEC and ATBC. However, when EC films were plasticized with GTA, the permeability decreased by at least 5 folds, to 0.127 g h-1 cm-3 mmHg-1 x 10-4 at 50 %RH and to 0.242 g h-1 cm-3 mmHg-1 x 10-4 at 75 %RH. From the analysis of plasticizer content in EC films stored at 50 %RH and 75 %RH over 4 weeks, it was found that GTA was lost from the films to the greatest extent (Table 2). It was therefore likely that during the conditioning of EC films for permeability study, a large amount of GTA was lost by degradation or through volatization. This would result in a re-alignment of the polymer chains and formation of a more compact structure which was less permeable to water vapour. Both plasticized Eudragit L 30D and EC films did not show any significant difference (p<0.05) in the permeability measured at 30°C, 75 %RH and 30°C, 50 %RH.
Conclusion
Free films prepared from aqueous ethylcellulose dispersions are weaker and more brittle compared to those prepared from acrylate. Due to its dense structure, MA film is less permeable and less likely to absorb moisture from the environment. This is attributed to its mechanical stability upon storage at high humidity. The stability of ethylcellulose films during storage is influenced by several factors, including type of plasticizers, plasticizer content, storage humidity and coalescence of polymer. Surelease films are more stable and less affected by ageing on storage at high humidity. The type of plasticizers may affect the mechanical properties of Aquacoat films on prolonged storage in three ways. Firstly, the concentration of unstable or volatile plasticizers such as GTA and DEP, may decrease during prolonged storage and produce more brittle films. Secondly, the type of plasticizers may affect the degree of coalescence or ageing of film on storage. Finally, the type of plasticizers also affects the influence of storage humidities on the mechanical properties of ethylcellulose films. Hence it is important to select carefully the type of plasticizers for film coating, as it may influence the stability of the final film coat.
References
Hutchings, D., Clarson, S., Sakr, A., Studies of the mechanical properties of free films prepared using an ethylcellulose pseudolatex coating system. Int J Pharm, 104(3): 203-213, 1994.
Aulton, M.E., Abdul Razzak, M.H., Hogan, J.E., Mechanical properties of hydroxypropylmethylcellulose films derived from aqueous systems. Part 1. Influence of plasticizers. Drug Dev Ind Pharm, 7(6): 649-668, 1981.
Wu, C., McGinity, J.W., Influence of relative humidity on the mechanical and drug release properties of theophylline pellets coated with an acrylic polymer containing methylparaben as a non-traditional plasticizer. Eur J Pharm Biopharm, 50(2): 277-284, 2000.
Chowhan, Z.T., Amaro, A.A., Chi, L.H., Comparative evaluations of aqueous film coated tablet formulations by high humidity aging. Drug Dev Ind Pharm, 8(5): 713-737, 1982.
Porter, S.C., Controlled-release film coatings based on ethylcellulose. Drug Dev Ind Pharm, 15(10): 1495-1521, 1989.
Iyer, U., Hong, W. M. N., Das, N., Ghebre-Sellassie, I., Comparative evaluation of three organic solvent and dispersion-based ethylcellulose coating formulations. Pharm Technol, 14: 68-86, 1990.
Bodmeier, R., Paeratakul, O., Mechanical properties of dry and wet cellulosic and acrylic films prepared from aqueous colloidal polymer dispersions used in the coating of solid dosage forms. Pharm Res, 11(6): 882-888, 1994.
Sadeghi, F., Ford, J.L., Rubinstein, M.H., Rajabi-Siahboomi, A.R., Comparative study of drug release from pellets coated with HPMC or Surelease. Drug Dev Ind Pharm, 26(6): 651-660, 2000.
Banker G.S., Film coating theory and practice. J Pharm Sci, 55: 81- 89, 1966.
Bodmeier, R., Paeratakul, O., Plasticizer uptake by aqueous colloidal polymer dispersions used for the coating of solid dosage forms. Int J Pharm, 152(1): 17-26, 1997.
American Society for Testing and Materials (ASTM). Designation: D 882-97: Standard test methods for tensile properties of thin plastic sheeting.
American Society for Testing and Materials (ASTM). Designation: E 96-95: Standard test methods for water vapour transmission of materials.
Wiederhold, P.R., (ed), Water Vapour Measurement: Methods and Instrumentation, Marcel Dekker, New York, 1997.
Binschaedler C., Gurny R., Doelker E., Influence of emulsifiers on film formation from cellulose acetate latexes experimental study of phase separation phenomena due to sodium dodecyl sulfate. I.. J Appl Polymer Sci, 34(8): 2631-2647, 1987.
Bindschaedler C., Gurny R., Doelker E., Influence of emulsifiers on film formation from cellulose acetate latexes. Modeling approach to the fate of emulsifiers in highly plasticized films. II. J Appl Polymer Sci, 37(1): 173-182, 1989.
Bilmeyer, F.W., (ed), Textbook of Polymer Science. Wiley, New York, pp. 246-247, 1984.
Parikh, N.H., Porter, S.C., Rohera, B.D., Tensile properties of free films cast from aqueous ethylcellulose dispersions. Pharm Res, 10(6): 810-815, 1993.
Rowe, R.C., Correlations between the in-situ performance of Tablet film coating formulations based on hydroxypropyl methylcellulose and data obtained from the tensile testing of free films. Acta Pharm Technol, 29: 205-207, 1983.
Surelease E-7-19010 - Product information Bulletin (Colorcon, US).
Pickard, J.F., (1979) Ph.D Thesis, CNAA.
Skultety, F. and Sims, S.M., Evaluation of the loss of propylene glycol during aqueous film coating. Drug Dev Ind Pharm, 13(12): 2209-2219, 1987.
Gutierrez-Rocca, J.C., McGinity, J.W., Influence of water soluble and insoluble plsticizers on the physical and mechanical properties of acrylic resin copolymers Int J Pharm, 103: 293-301, 1994.
Onions, A., Films from water-based colloidal dispersions. Manufacturing chemist 12: 55-59, 1986.
Keshikawa, T., Nakagami, H., Film formation with coating systems of aqueous suspensions and latex dispersions of ethylcellulose. Chem Pharm Bull, 42(3): 656-662, 1994.
Sun, Y.M., Huang, W.F., Chang, C.C., Spray-coated and solution-cast ethylcellulose pseudolatex membranes. J Membrane Sci, 157(2): 159-170, 1999.
Guo, J.H., Robertson, R.E., Amidon, G.L., An investigation into the mechanical and transport properties of aqueous latex films: a new hypothesis for the film-forming mechanism of aqueous dispersion system. Pharm Res, 10(3): 405-410, 1993.
Guo, J.H., Robertson, R.E., Amidon, G.L., Influence of physical aging on mechanical properties of polymer free films: The prediction of long-term aging effects on the water permeability and dissolution rate of polymer film-coated tablets. Pharm Res, 8(12) 1500-1504, 1991.
Corresponding Author: Paul W.S. Heng, Department of Pharmacy, Faculty of Science, National University of Singapore, Singapore. phapaulh@nus.edu.sg
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps