J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(3):360-362, 2003
The antinociceptive activities of 1-(4-aryl-2-thiazolyl)-3,5-disubstituted-2 pyrazolines in mouse writhing test.
Abbas Shafiee, Maryam Bagheri, Maryam Shekarchi
Department of Medicinal Chemistry, Faculty of Pharmacy, and Laboratory of Chemistry, Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, IranMohammad Abdollahi1
Department of Toxicology and Pharmacology, Faculty of Pharmacy, and Laboratory of Toxicology, Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, IranReceived 13 September 2003, Revised 12 December 2003, Accepted 12 December 2003
PDF version
Abstract
PURPOSE: Sympathetic nervous system stimulation, which releases noradrenalin, influences the nociceptive activity that develops after tissue injury. The a2-adrenergic agonist, clonidine, produces analgesia through a central mechanism but also inhibits noradrenalin release at terminal nerve fiber endings. METHODS. In this study the effects of some 1-(4-aryl-2-thiazolyl)-3,5-disubstituted-2-pyrazolines (compounds 1a-1e) as analogues of clonidine with some modifications were studied by writhing test, a visceral pain model in mice. RESULTS. Compounds 1a and 1c induced significant reduction in writhing response when compared to control. Regarding the dose-response relationship of compounds 1a and 1c, it is evident that the activity of these compounds is in direct proportion to their dose and all compounds show activities comparable to clonidine. In addition compounds 1a and 1c, having methoxy and nitro groups respectively at para position of 4-aryl showed better antinociception than compounds having similar groups in meta position namely 1b and 1e. This might be due to better interaction of these compounds with the a2-receptors. CONCLUSIONS. The antinociceptive properties of these newly synthesized compounds is comparable to clonidine. Further studies are needed to explore the differences in the efficacy and safety of synthesized compounds.
Introduction
Alpha-2-Adrenergic agonists, like clonidine are supposed to produce analgesia mainly through an inhibition of the transmission of nociceptive stimulation, in the dorsal horn of the spinal cord [1]. Clonidine is reported to mimic the effect of noradrenalin release by descending inhibitory nociceptive control pathways [2]. Topical administration of clonidine may also decrease pain intensity in patients with sympathetically maintained pain [3], suggesting a peripheral site of action for this drug. Recently it has been reported that a modified form of clonidine, thiazolo imidazoline, has better antihypertensive activity than clonidine, which promoted us to synthesis and examine these new compounds. The previous studies on the antihypertensive activities of several 1-(2-thiazolyl)-3,5-disubstituted-2-pyrazolines revealed their antihypertensive as well as clonidine [4,5]. The present study reports the antinociceptive effects of these compounds.
Materials And METHODS
Chemistry
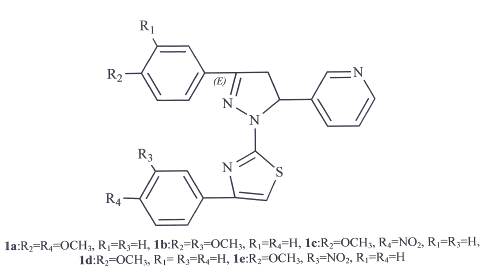
Compounds 1a-1e (1a:R2=R4=OCH3, R1=R3=H, 1b:R2=R3=OCH3, R1=R4=H, 1c:R2=OCH3, R4=NO2, R1=R3=H, 1d:R2=OCH3, R1= R3=R4=H, 1e:R2=OCH3, R3=NO2, R1=R4=H) were synthesized as described previously [4] by reacting 3-(4'-methoxyphenyl)-5-(3'-pyridyl)-1-thiocarbamoyl-2-pyrazoline with phenacyl bromide or its derivatives (R3=H, OCH3, NO2, R4=H, OCH3, NO2) in ethanol and purified with ethanol.These new compounds were confirmed by 1HNMR, Mass spectral and Elemental analysis (figure 1).The purity of all compounds were confirmed by TLC using different mobile phases.
Pharmacology
Animals
Male albino NMRI mice from Pasture Institute of Tehran weighting 20-25 g were used in this study. Mice were housed in colony cages and were given a standard laboratory chow and water ad libitum. A 12h /12h light-dark cycle was used, with all the treatment being performed during the light period of the cycle. Both the animal unit and laboratory temperature were maintained at 22±2°C. The mice were allowed to habituate to the laboratory environment for 2 h before the experiments were initiated. All ethical manners for use of laboratory animals were considered carefully.
All compounds were suspended in water and tween 80 (1% w/v) and administered intraperitoneally (IP) at 2 mg kg-1 in 0.1 ml volume. Clonidine (0.1 mg kg-1) was used as a reference drug. The control group received vehicle (1 ml kg-1, IP) alone. To obtain dose response relationship, doses of 1, 2 and 4 mg kg-1 of compounds 1a and 1c were also tested.
Writhing Test
All treatments were done 30 minutes before IP administration of acetic acid 0.7% (17.5 mg kg -1) (1 ml kg -1). Animals were individually housed in a glass cylinder on a flat glass and a mirror was arranged at an angle of 45° under the cylinder. Antinociception was recorded by counting the number of writhings immediately after injection of acetic acid during 30 minutes. A writhe is indicated by abdominal constriction and stretching of at least one hind limb [6, 7].
Statistical Analysis
Comparison between groups was made by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. Differences with P<0.05 between experimental groups were considered statistically significant.
Results
Antinociceptive effects induced by different doses of test compounds on mouse writhing test are shown in table 1.
Table 1: Effects of compound a-e on acetic acid-induced mouse writhing test.
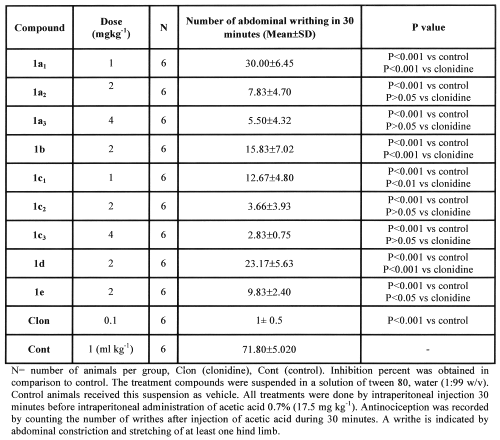
Compounds (1a-1e) at dose of 2 mg kg-1 induced significant reduction in writhing response in comparison to control as follow: (1a: 87%, P<0.001), (1b: 78.2%, P<0.001), (1c: 95.2%, P<0.001), (1d: 68.3%, P<0.001), (1e: 86.5%, P<0.001).
Since compounds 1a and 1c showed the best response when compared to clonidine (table 1), thus additional doses of 1, 2 and 4 mg kg-1 were also tested. They induced antinociception in comparison to control as follow: (1a1: 59%, P<0.001), (1a2: 87%, P<0.001), (1a3: 92.4%, P<0.001), (1c1: 82.8%, P<0.001), (1c2: 95.2%, P<0.001), (1c3: 96.5%, P<0.001). The antinociception activities of 1a and 1c were dose-dependent (r2=0.9197 and r2=0.9145 respectively).
Discussion
Overall results of the present study show good antinociceptive effects of tested compounds in comparison to control and clonidine. Supporting the present results, there is evidence that subcutaneous administration of clonidine 0.05 mg kg-1 induces complete antinociception in rat writhing test after injection of phenylquinine [8]. The author concluded that clonidine-induced antinociception is mediated centrally. In addition, there is evidence that when pain is evoked by an inflammatory stimulus such as in the phenylquinine writhing test, clonidine as well as several other a-adrenoceptor agonists including those which do not cross the blood brain barrier, promote analgesia [9] suggesting that, in addition to well known centrally mediated analgesic action, clonidine could also promote antinociception through a peripheral mechanism. In addition, there is evidence that drugs such as clonidine could induce peripheral antinociception by a-adrenoceptor-mediated local release of enkephalin-like substances [10]. When administered intra-articular, clonidine like morphine can produce analgesia in patients submitted to knee surgery [11].
In writhing test both centrally and peripherally, mediated analgesia can be detected and several mediators such as kinin, acetylcholine, substance P and prostaglandins take part in visceral pain model nociception [7] and transmission of nociception from the viscera [12].
Regarding the dose-dependent antinociception of compounds 1a and 1c, it is evident that the activity of these compounds is in direct proportion to their dose with r2=0.9197 and r2=0.9145 respectively. All compounds show activities comparable to clonidine. Considering the structure activity relationship of tested compounds the existence of bulk groups at R2 and R4 positions (figure 1) might have improved the pharmacological effects.
Figure 1: The chemical structure of compounds 1a-1e.
In addition, these substitutions may cause increase in lipophilicity and totomerisem. As a result, these compounds might pass easier and faster from blood brain barrier. The compounds 1a and 1c, having methoxy and nitro groups at para position of aryl respectively showed better antinociception than compounds having similar groups in meta position namely 1b and 1e. As shown in table 1, compounds having methoxy and nitro groups at para or meta positions, showed better antinociception than other tested ones. This might be also explained by probable better interaction of these compounds with the αa2-receptors, which remains to be elucidated later.
An accurate estimation of ED50 was not possible due to the close response values.
Finally, it is concluded that the antinociceptive properties of these newly synthesized compounds are comparable to that of clonidine. Further studies are needed to explore the differences in the efficacy and safety of synthesized compounds.
REFERENCES
Yaksh TL, Reddy SV. Studies in the primate on the analgetic effects associated with intrathecal actions of opiates, alpha-adrenergic agonists and baclofen. Anesthesiology 1981;54(6):451-67.
Yeomans DC, Clark FM, Paice JA, Proudfit HK. Antinociception induced by electrical stimulation of spinally projecting noradrenergic neurons in the A7 catecholamine cell group of the rat. Pain 1992;48(3):449-61.
Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with mpathetically maintained pain. Pain 1991;47(3):309-17.
Bagheri M, Shekarchi M, Jorjani M, Ghahremani M.H, Vosooghi M, Shafiee A. Synthesis and Antihypertensive Activity of 1-( 2-thiazolyl)-3,5-disubstituted -2-pyrazolines. Arch Pharm Pharm Med Chem 2003 (in press).
van Zwieten PA. Centrally induced hypotension by 2-(2,6-dichlorophenyl)-5,6-dihydroimidazo (2,1-b)-thiazole fumarate (compound 44-549). Pharmacology. 1975;13(4):352-5.
Jain SC, Jain R, Sharma RA, Capasso F. Pharmacological investigation of Cassia italica, J Ethnopharmacol 1997;58:135-142.
Vogel HG, Vogel WH. Drug Discovery and Evaluation, Pharmacological assays, Berlin Heidelberg: Springer-Verlag, 1997.
Fielding S, Spaulding TC, Lal H. Antinociceptive actions of clonidine. Prog Clin Biol Res 1981;71:225-42.
Bentley GA, Copeland IW, Starr J. The actions of some alpha-adrenoreceptor agonists and antagonists in an antinociceptive test in mice. Clin Exp Pharmacol Physiol. 1977;4(4):405-419.
Nakamura M, Ferreira SH. Peripheral analgesic action of clonidine: mediation by release of endogenous enkephalin-like substances. Eur J Pharmacol 1988; 146(2-3):223-8.
Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991; 17;325(16):1123-6.
Cervero F, Laird JM. Visceral pain. Lancet 1999; 353(9170):2145-8.
Corresponding Author: Mohammad Abdollahi, Laboratory of Toxicology, Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, Iran. mohammad.abdollahi@utoronto.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps