J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(2):238-245, 2003
Poly n-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: in vitro release and in vivo skin penetration.
Shozo Miyazaki, Akie Takahashi, Wataru Kubo
Faculty of Pharmaceutical Sciences, Health Sciences University of Hokkaido, Ishikari-Tohbetsu, Hokkaido, JapanJohn Bachynsky, Raimar Löbenberg1
Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, CanadaReceived 28 April 2003, Revised 30 April 2003, Accepted 16 June 2003
PDF version
Abstract
Purpose: The aim of this work was to prepare poly n-butylcyanoacrylate (PNBCA) nanocapsules loaded with indomethacin and to evaluate the ability of this carrier system to deliver the drug systemically after its topical application. METHODS: Poly n-butylcyanoacrylate (PNBCA) nanocapsules of indomethacin were prepared by interfacial polymerization. The physicochemical characterization of the PNBCA nanocapsules was performed by measuring the drug content by HPLC and analyzing the particle size using scanning electron microscopy. The in vitro permeation of indomethacin through excised rat skin and an artificial membrane was determined for PNBCA nanocapsules in pH 7.4 phosphate buffer (I), and in PLF-127 gel (II) and were compared against indomethacin incorporated into 25%w/w PLF-127 gel alone (III). The in vivo percutaneous absorption of indomethacin following the application of the PNBCA nanocapsules and a 25%w/w Pluronic F-127 (PLF-127) gel (III) was monitored by the determination of drug plasma levels in rats. RESULTS: The drug loading results indicated that ~76.6% of indomethacin was loaded onto the PNBCA nanocapsules; the average particle size was 188 nm. The in vitro results indicated a rank order for the three formulations (I, II and III) in both the flux at steady state and the cumulative amounts permeated at 8 hrs. The higher drug plasma levels over 6 hrs of indomethacin PNBCA nanocapsules are in agreement with the determined in vitro permeation results. CONCLUSION: The presented data show that indomethacin loaded PNBCA nanocapsules can improve the transdermal delivery of indomethacin compared to a conventional gel formulation using Pluronic F-127. This might be due to their ultra fine particle size and their hydrophilic and hydrophobic surface characteristics.
Introduction
In recent years, colloidal drug delivery systems such as liposomes and nanoparticles have been extensively studied as one of the most promising strategies to achieve site-specific drug delivery (1). More recently, nanocapsules formed from lipophilic droplets, as the core, surrounded by a thin wall of polymeric material prepared by anionic polymerization of alkylcyanoacrylate monomer have been proposed as vesicular colloidal polymeric drug carriers (1-11). Polyalkylcyanoacrylate based nanoparticles have been extensively investigated for oral (12, 13) and ocular (14) administration. The oral use of colloidal carrier systems for peptide delivery and especially as adjuvant for oral vaccination seems to be a promising application for such carrier systems (15). Colloidal carrier systems of poly-alkylcyanoacrylate nanoparticles are one of many drug delivery systems that have been proposed for improving the poor ocular bioavailability of ophthalmic medications (16).
Furthermore, colloidal carriers could be useful to deliver several drugs into the skin and the stratum corneum. This might cause a drug supply to the skin over a prolonged time period. A sustained drug release might reduce systemic drug absorption and a local treatment of inflammation might reduce systemic side effects. Because of their ultra fine particle size and their oily vesicular nature, alkylcyanoacrylate nanocapsules can sustain drug release, and, as a result, this colloidal carrier system has great potential as a topical controlled drug delivery system. However, their utility in topical drug delivery has been questioned. As nanoparticles can cross the eye corneal epithelium, it would be interesting to investigate the ability of nanoparticles to pass through the skin (17). If nanoparticulate carriers can across the stratum corneum then they can act as micro-reservoirs of a drug in the horny layer and provide a sustained drug delivery. However, no specific information on the uptake of poly-alkylcyanoacrylate nanoparticles by corneocytes has yet been presented.
In previous publications (18, 19), thermally reversible gels of Pluronic F-127 were evaluated as vehicles for the percutaneous administration of NSAIDs (indomethacin). Therefore, a drug delivery to the skin could possibly be realized if this colloidal carrier system of indomethacin is incorporated into a Pluronic F-127 gel formulation.
The aim of this work was to prepare poly n-butylcyanoacrylate (PNBCA) nanocapsules of indomethacin, and to incorporate them into a Pluronic F-127-based gel to evaluate its ability to deliver the drug systemically after its topical application.
Materials and Methods
Materials
Indomethacin, Pluronic F-127 and Pluronic F-68 were purchased from Sigma Chemicals (Saint Louis, MO, USA). The n-butylcyanoacrylate monomer (Lot.02GD9236) used to prepare the nanocapsule membrane was a gift from Loctite Ltd. (Dublin, Ireland). Phospholipid (first grade soybean lecithin) was obtained from Wako Pure Chemical Industries (Tokyo). All other chemicals and solvents used were reagent grade.
Nanocapsule preparation
The indomethacin-loaded PNBCA nanocapsules were prepared according to a method described previously (12, 20) with slight modifications. An alcoholic solution (100 mL) containing the monomer, indomethacin, benzyl benzoate and phospholipids was slowly injected through a syringe with a needle into an aqueous solution (200 mL) containing the poloxamer and subjected to magnetic agitation at 600 rpm. The nanocapsules were formed by interfacial polymerization over 10 hours. The resulting colloidal suspension was concentrated by rotor evaporation under vacuum at 40°C to a final volume of 40 mL, and then filtered through sintered glass (10-16 mm). A typical composition of a nanocapsules suspension in the final form consisted of indomethacin 0.05g, n-butylcyanoacrylate 0.5 mL, benzyl benzoate 2 mL, phospholipid 1.0 g, Pluronic F-68 1.0 g and water to 40.0 mL.
Determinations of drug content
An ultracentrifugation technique (21) was used to separate the free drug from the nanocapsules and to estimate the drug loading of the nanocapsules. The final colloidal suspensions were ultracentrifuged at 80.000 g at 4°C for 1.5 hrs (Hitachi SCP 70H2, Tokyo, Japan). The drug concentration was determined by high-performance liquid chromatography as described previously (22) after dissolving the nanocapsule sediment in chloroform.
Morphological analysis
For morphological examinations, nanocapsules were analyzed with a scanning electron microscopy (Hitachi type X-650, Tokyo, Japan). Photographs were taken after being fixed on a sample support and gold metallized.
In vitro permeation study
The in vitro permeation of indomethacin was studied by using a plastic diffusion cell similar to that described previously (23). The capacity of each half-cell was 2 mL and the surface area of the membranes was 2.0 cm 2 . After the ultracentrifugation, the nanocapsule formulations were dispersed in pH 7.4 phosphate buffer (I) or PLF-127 gel (II) and placed into the donor compartment. All permeation studies were performed at 37°C. An equal volume of the pH 7.4 phosphate buffer was placed into the receptor compartment. The donor and the receptor compartments were separated by a freshly-excised, full-thickness, piece of rat skin or an artificial cellulose membrane with an average pore size of 50Å (Viskase Sales Co., Chicago, USA). The assembled cells were shaken horizontally at the rate of 60 strokes min -1 in an incubator. The total volume of the receptor solution was removed at predetermined intervals throughout the experiment and replaced by fresh medium. The drug concentrations were determined by high-performance liquid chromatography as described previously (22).
Confocal laser scanning microscopy (CLSM)
The skin distribution of rhodamine-loaded poly-iso-butylcyanoacrylate nanoparticles was determined by CLSM. Nanoparticles were chosen over nanocapsules for this experiment to ensure that no fragments or possibly ruptured capsules were detected in the skin. The particles were prepared by an emulsion polymerization method. 500 mL of a rhodamin 6G stock solution (0.1 mg/mL) (Sigma, St. Louis) was added to 10 mL of a 0.01 N hydrochloric acid solution containing 50 mg of Pluronic F68 (BASF, Ludwigshafen) as emulsifier. 100 mL of monomer were added drop wise to the solution under constant stirring at 600 rpm. The nanoparticle suspension was neutralized after 4 hours using 10 mL of a 1 N sodium hydroxide solution. The particles were purified from unbound dye and polymerization residuals using centrifugation.
The nanoparticle size was measured using a Zetasizer (Zetasizer 3000HSA, Malvern Instruments). The mean particle size of the resulted nanoparticles was 132.7 nm +/- 13.1 with a polydispersity index of 0.213 (the extent of the rhodamine 6G encapsulation was not determined).
Vertical slicing of frozen skin after an in vitro skin permeation study at 2 hours was used to study the skin distribution of the fluorescent-labeled nanoparticles and a control solution. The skin was removed from the diffusion cells and frozen in a bedding medium containing 2% methylcellulose (Methocel, Dow Chemicals). The orientation of the skin in the bedding medium was fixed in a way that vertical cross-sections of the rat skin could be cut. The slicing was performed in a way which prevented any carryover of the fluorescent label from the stratum corneum to the dermis or subcutaneous tissues. The skin was cut in a cyo-microtom. 25 mm thick tissue slices were cut and transferred to glass slides. The slices were observed with a Confocal Laser Scanning Microscopy (CLSM) (Leica TCS-SP2 Multiphoton Confocal Laser Scanning Microscopy). The stratum corneum, epidermis, dermis and subcutaneous tissues were identified in each tissue slice. Representative pictures were taken.
Animal experiments
Male Wistar rats weighing 280-380 g were used in the in vitro permeation and in vivo percutaneous absorption studies.
The Animal Ethics and Research Committee of the Health Sciences University of Hokkaido previously approved the protocols for the animal experiments.
The experiments were performed in a constant temperature room (21-22°C). The day before the experiment the hair of the abdominal parts was carefully removed with an electric clipper and a razor without breaking the skin.
The rats were anaesthetized with an i.p. injection of urethane, 1 g/kg, and the jugular vein was cannulated to facilitate the removal of blood samples. The animals were kept anaesthetized over the duration of the experiment to avoid that they can remove the applied formulations. A portion of nanocapsules containing 10 mg indomethacin were separated by ultracentrifugation from the suspension as described above in Section 2.2 and applied to a 3-cm diameter circular site, (surface area 7.1 cm 2 ), on the abdominal skin. After drug administration, blood samples were collected from the jugular vein at hourly intervals and centrifuged at 11750 g for 3 min.
The drug plasma concentration was determined by high-performance liquid chromatography. The area under the plasma concentration curve (AUC), up to 6 h post administration, was calculated by moment analysis (24).
Results and discussion
Characteristics and drug content of nanocapsule
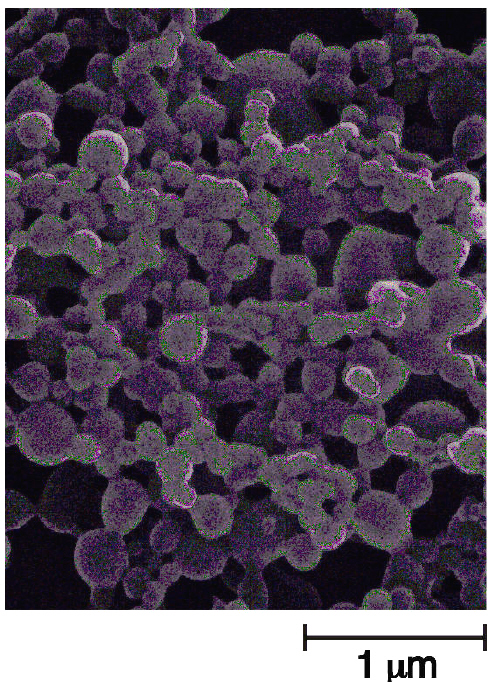
Figure 1 shows a scanning electron micrograph of PNBCA nanocapsules containing indomethacin prepared by interfacial polymerization.
Figure 1: Scanning Electron Micrograph of indomethacin-loaded PNBCA nanocapsules.
The PNBCA nanocapsules were spherical with a smooth surface. The sizes of 50 nanocapsules were determined from the photographs. The resulting nanocapsules were in the size range of 100-360 nm, with a mean diameter of 188±7 nm (mean±S.E. n=50). The drug content analysis showed that about 76.6%±1.2%(mean±S.E. n=8) of the total indomethacin concentration was encapsulated in the PNBCA nanocapsules.
In vitro permeation through excised rat skin
In order to study the permeation of PNBCA nanocapsules through the rat skin, the nanocapsule formulations containing 0.5%w/v indomethacin were dispersed in a pH 7.4 phosphate buffer (I) or 25%w/w a PLF-127 gel(II) and placed in the donor compartment and the permeation experiment was carried out. The control experiment was 0.5%w/v indomethacin incorporated into 25%w/w PLF-127 gel (III).
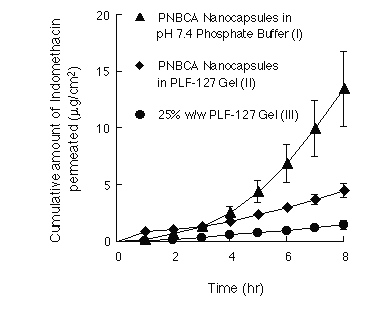
The cumulative drug amounts permeated through the rat skin were plotted against the time as shown in Figure 2.
Figure 2: Cumulative amount of indomethacin (initial loading 0.5% w/v) per unit area, permeating through excised rat skin when released from PNBCA nanocapsules dispersion in pH 7.4 phosphate buffer, PNBCA nanocapsules dispersion in Pluronic F-127 gel and 25% w/w Pluronic F-127 gel. Each value is the mean ±S.E. of 4 determinations.
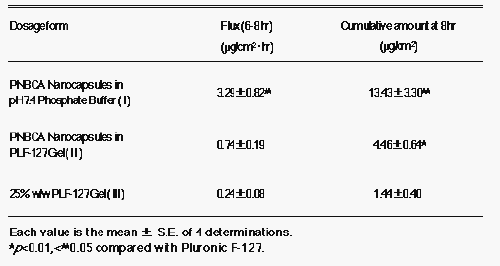
The plots were linear after an initial lag time. The skin permeation parameters of indomethacin are listed in Table 1.
Table 1: In vitro permeation studies: Flux and cumulated amount of indomethacin through rat skin from different formulations.
The results indicated a rank order for all three formulations (I, II and III) in regard to both the flux at steady state and the cumulative amounts permeated at 8 hrs. The flux of formulation I at steady state (3.29 mg/cm 2 hr) and the cumulative amount at 8 hrs (13.43 μg/cm 2 ) were about 13.8 and 9.3 times greater than those of formulation III (0.24 μg/cm 2 hr and 1.44 μg/cm 2 ), respectively. The nanocapsules of indomethacin, when dispersed in 25%w/w PLF-127 gel (formulation II), showed a smaller flux and cumulative amounts, due to the viscous environment provided by the PLF-127 gel. These results suggested that PNBCA nanocapsules are able to permeate through rat skin in a period of 8 hrs.
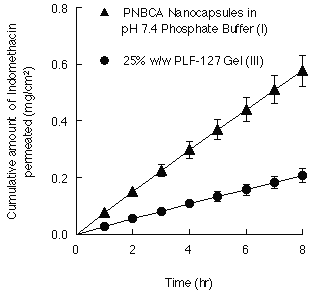
Figure 3: Cumulative amount of indomethacin (initial loading 0.5% w/v) per unit area, permeating through cellulose membranes when released from PNBCA nanocapsules dispersion in pH 7.4 phosphate buffer and 25% w/w Pluronic F-127 gel. Each value is the mean ±S.E. of 3 determinations.
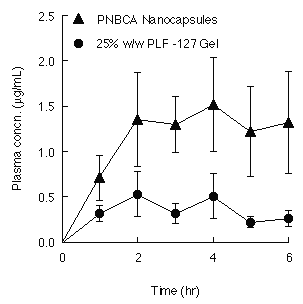
Figure 4: Percutaneous absorption in rats of indomethacin from PNBCA nanocapsules and 25% w/w Pluronic F-127 gels loaded with an initial drug concentration of 1% w/v. Each value is the mean ±S.E. of 6 determinations.
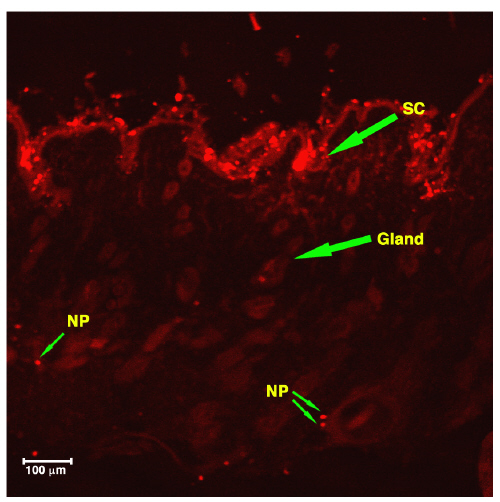
Figure 5 shows a CLMS picture of rat skin tissue 2 hrs after the treatment with nanoparticles.
Figure 5: Fluorescence photomicrograph of vertical slicing of rat skin after application of Rhodamine loaded PIBCA NP for 2 hours. SC. Stratum Corneum: NP. Nanoparticles.
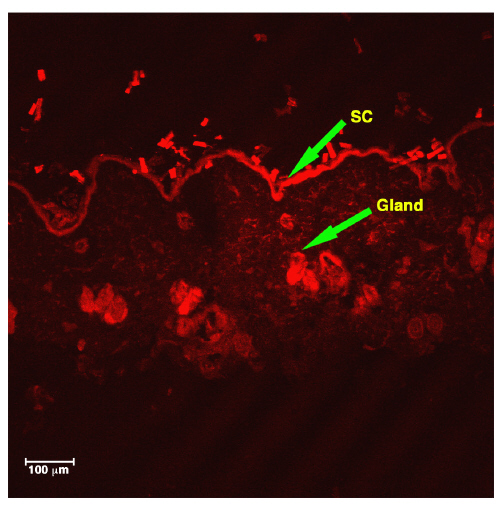
The nanoparticles were clearly visualized in the stratum corneum, epidermis and dermis. When a rhodamine solution was applied to the skin (Figure 6), only the red background corresponding to the free probe was observed.
Figure 6: Fluorescence photomicrograph of vertical slicing of rat skin after application of Rhodamine solution for 2 hours. SC. Stratum Corneum: NP. Nanoparticles.
These results revealed that nanoparticles can penetrate through the stratum corneum and reach the epidermis.
The in vitro release of indomethacin was negligible when the pH in the receptor compartment was kept below pH 5(25). At physiological pH 7.4, at which indomethacin becomes water-soluble, the release was slow and incomplete as a result of drug partition between the inner lipophilic phase of nanocapsules and the aqueous phase.
The influence of the in vitro release of indomethacin at pH 7.4 from PNBCA nanocapsules was investigated using a cellulose membrane. The nanocapsules with a mean diameter of 188 nm used in this study were not able to permeate through the cellulose membranes with an average pore size of 5 nm; the permeation of the drug from the donor to the acceptor compartment was only induced by the free drug released from the nanocapsules.
The cumulative amounts of the drug permeation through a cellulose membrane were plotted against the time as shown in Fig. 3. A linear relationship was obtained for two formulations; PNBCA nanocapsules dispersed in a pH 7.4 phosphate buffer (I) and 25%w/w PLF-127 gel (III), showing that the permeations were well described by zero-order kinetics. Release rates calculated from the gradients of the linear plots were compared and show significantly higher values when indomethacin was released from PNBCA nanocapsules (formulation I, 7.2 ±0.7x10 -2 mg/cm 2 hr, mean ±S.E., n=3) compared to its release from the gel formulation of PLF-127 (formulation III, 2.6 ±0.3x10 -2 mg/cm 2 hr). Only ~ 12.4% of the initial amount of the encapsulated drug was released within 8 hrs from the PNBCA nanocapsules.
These results indicated that the indomethacin release from the nanocapsules in the donor compartment was not sufficient to account for the total appearance of the drug in the acceptor compartment when rat skin was used. The permeation of the drug through rat skin must mostly be induced following the permeation of the intact nanocapsules.
In vivo percutaneous absorption in rats
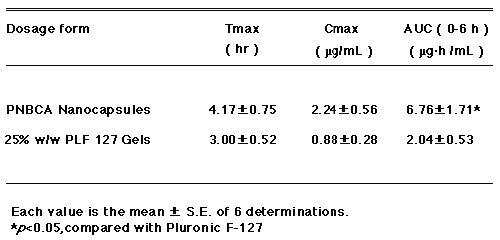
The in vivo percutaneous absorption of indomethacin, following the application of the intact PNBCA nanocapsules and the 25%w/w PLF-127 gel (III) containing 1%w/v drug to a defined area of the abdominal rat skin, was monitored by the determination of drug plasma levels. Fig. 4 compares the plasma concentrations achieved following the application of each formulation up to 6 hrs post-administration. Table 2 summarizes the AUC values calculated from the plasma concentration-time data using a model-independent analysis (24).
Table 2: Comparison of bioavailability parameters.
The higher indomethacin plasma levels over 6 hrs following the application of intact PNBCA nanocapsules is in agreement with the in vitro permeation results, which show a higher permeation rate for this drug through excised rat skin compared to that of a 25%w/w PLF-127 gel (III). The higher plasma concentrations using nanoparticles result in an increase in the AUC values by factor 3.3 compared to the AUC values of the Pluronic F-127 gel formulation.
The topical application of nanocapsules on the skin surface was observed over the duration of the experiment and compared with the PLF-127 gel (figure not shown). When the PLF-127 gel was applied to the skin, it formed a thin, smooth film on the skin surface. This is due to the evaporation of water from the gel. The lower drug plasma concentrations observed with the PLF-127 gel may be due to this evaporation effect, but might be also attributed to a high affinity of the drug to the hydrophobic domains of the PLF-127. However, an interesting observation was that the intact nanocapsule formulation gradually disappeared from the skin surface over time.
These results suggested that the PNBCA nanocapsules could penetrate through the stratum corneum and the epidermis and reach the blood circulation. This observation is due to their ultra fine particle size and their hydrophilic and hydrophobic surface characteristics. However, the penetration mechanism of the nanocapsules through the skin is not known and requires more research. Previous papers have explored the use of microparticles for topical application. Rolland et al. (26) reported that adapalene loaded microspheres (5 mm mean diameter) were specially targeted to the follicular ducts and did not penetrate via the stratum corneum. Recently, Jaron et al. (27) showed that the PLGA microparticles (1-10 mm) could effectively penetrate into the porcine skin through the stratum corneum and reach the epidermis, although the larger particles remained on the skin surface.
The PNBCA nanocapsule carriers described in this work can be used as vehicles for topical drug delivery, in order to improve the skin permeation of drugs such as indomethacin and presumably other more hydrophobic drugs. The results showed that the application of the nanoparticles increased the systemic availability of indomethacin and did not reduce it as was initially expected. It is suggested that a rapid onset of a pharmacological effect is sufficiently induced by free indomethacin in the skin and plasma followed by the absorption of the intact nanocapsules. The increased skin permeation of indomethacin may be explained by a modification of the lipid organization in the skin due to the presence of nanocapsules. Indeed some of the nanocapsule components (benzyl benzoate) are known to act as absorption enhancers. Further studies are necessary to evaluate if indomethacin loaded nanocapsules can be used for the systemic or local treatment of diseases like arthritis and other inflammatory conditions.
Conclusion
The presented data show that indomethacin loaded PNBCA nanocapsules can improve the transdermal delivery of indomethacin compared to a conventional gel formulation using Pluronic F-127. The in vitro and in vivo evaluation of the PNBCA nanocapsules indicate that the increased drug concentrations measured in the plasma are due to the penetration of intact nanocapsules through the rat skin. Colloidal drug carriers such as nanocapsules are promising delivery systems for dermal formulations of drugs and have a high potential for local and presumably systemic drug delivery of anti-inflammatory substances such as indomethacin. This has important implications for the treatment of diseases like arthritis.
Acknowledgements
This study was supported in part by the Thaiho Endowed Research Fund of the University of Alberta. The authors are grateful to Dr. N. Swords of Loctite Biomedical (Ireland) Ltd. for the supply of n-butylcyanoacrylate.
References
Kreuter J., Nanoparticles. In Colloidal drug delivery systems. Kreuter J.(Eds.) Marvel Dekker, New York, p219-342, 1994.
Couvreur, P., Kante, B., Roland, M., Guiot, P., Baudin, P., Speiser, P., Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. J. Pharm. Pharmacol. 31, 331-332, 1979.
Couvreur, P., Kante, B., Roland, M., Speiser, P., Adsorption of antineoplastic drugs to polyalkylcyanoacrylate nanoparticles and their release in calf serum. J. Pharm. Sci. 68, 1521-1524, 1979.
Couvreur, P., Kante, B., Lenaerts, V., Scailteur, V., Roland, M., Speiser, P., Tissue distribution of anticancer drugs associated to polyalkylcyanoacrylate nanoparticles. J. Pharm. Sci. 69,199-202, 1980.
Kante, B., Couvreur, P., Lenaerts, V., Guiot, P., Roland, M., Baudhuin P., Speiser, P., Tissue distribution of 3H-actinomycin D absorbed on polybutyl-cyanoacrylate nanoparticles. Int. J. Pharm. 7, 45-53, 1980.
Couvreur, P., Kante, B., Grislain, L., Roland, M., Speiser, P., Toxicity of polyalkylcyanoacrylate nanoparticles. II. Doxorubicin loaded nanoparticles. J. Pharm. Sci. 71, 790-793, 1982.
Kreuter, J., Mills, S., N., Davis, S. S., Wilson, C. G., Polybutylcyanoacrylate nanoparticles for the delivery of [75Se] norcholesterol. Int. J. Pharm. 16, 105-113, 1983.
El-Samaligy, M. S., Rohdevald, P., Mahmoud, H. A., Polyalkyl cyanoacrylate nanocapsules. J. Pharm. Pharmacol. 31, 216-218, 1986.
Damge, C., Michel, C., Aprahamian, M., Couvreur, P., New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 37, 246-251, 1988.
Allemann, E., Gurny, R., Doelker, E., Drug-loaded nanoparticles preparation methods and drug targeting issues. Eur. J. Pharm. Biopharm. 39, 173-191, 1993.
Das, S. K., Tucker, I. G. Hill, D. J., Ganguly, N., Evaluation of poly(isobutylcyanoacrylate) nanoparticles for mucoadhesive ocular drug delivery. I. Effect of formulation variables on physicochemical characteristics of nanoparticles. Pharm. Res. 12, 534-540, 1995.
Andrieu, V., Fessi, H., Dubrasquet, M., Devissaguet, J-Ph., Puisieux, F., Benita, S., Pharmacokinetic evaluation of indomethacin nanocapsules. Drug Des. Del. 4, 295-302, 1989.
Araujo, L., Sheppard, M., Lobenberg, R., Kreuter, J., Uptake of PMMA nanoparticles from the gastrointestinal tract after oral administration to rats: Modification of the body distribution after suspension in surfactant solutions and in oil vehicles. Int. J. Pharm. 176, 209-224, 1999.
Damge, C., Aprahamian, M., Balboni, G., Hoeltzel, A., Andrieu, V., Devissaguet, J-Ph., Polyalkylcyanoacrylate nanocapsules increase the intestinal absorption of a lipophilic drug. Int. J. Pharm. 36, 121-125, 1987.
Jung, T., Kamm, W., Breitenbach, A., Kaiserling, E., Xiao, JX., Kissel, T., Biodegradable nanoparticles for oral delivery of peptides: Is there a role for polymers to affect mucosal uptake? Eur. J. Pharm. Biopharm. 50 147-160, 2000.
Harima, T., Kreuter, J., Speiser, P., Boye, T., Gurny, R., Kubis, A., Enhancement of the myotic response of rabbits with pilocarpine-loaded polybutyl-cyanoacrylate nanoparticles. Int. J. Pharm. 33, 187-193, 1986.
Alonso M.J., Nanoparticulate drug carrier technology. In Microparticulate systems for the delivery of proteins and vaccines. Cohen S. and Bernstein H. (Eds.) Marvel Dekker, New York, p203-242, 1996.
Miyazaki, S., Tobiyama, T., Takada, M., Attwood, D., Percutaneous absorption of indomethacin from pluronic F-127 gels in rats. J. Pharm. Pharmacol. 47, 455-457, 1995.
Takahashi, A., Suzuki, S., Kawasaki, N., Kubo, K., Miyazaki, S., Loebenberg, R., Bachynsky, J., Attwood, D., Percutaneous absorption of non-steroidal anti-inflammatory drugs from in situ gelling xyloglucan formulations in rats, Int. J. Pharm., 246, 179-186, 2000.
Al Khouri Fallouh, N., Roblot-Treupel, L., Fessi, H., Devissaguet, J-Ph., Puisieux, F., Development of a new process for the manufacture of polyisobutyl-cyanoacrylate nanocapsules. Int. J. Pharm. 28, 125-132, 1986.
Fawaz, F., Guyot, M., Lagueny, A.M., Devissaguet, J.Ph., Ciproflexan-loaded polyisobutylcyanoacrylate nanoparticles: preparetion and characterization. Int. J. Pharm. 154, 191-203, 1997.
Miyazaki, S., Yokouchi, C., Nakamura, T., Hashiguchi, N., Hou, W.-M., Takada, M., Pluronic F-127 gels as a novel vehicle for rectal administration of indomethacin. Chem, Pharm. Bull. 34, 1801-1808, 1986.
Miyazaki, S., Takeuchi, S., Yokouchi, C., Takada, M., Pluronic F 127 gels as a vehicle for topical administration of anticancer agents. Chem. Pharm. Bull. 32, 4205-4208, 1984.
Yamaoka, K., Tanigawa, Y., Nakagawa T., Uno, T., 1981. Pharmacokinetic analysis program (MULTI) for microcomputer. J. Pharmacobio-Dyn. 4, 879-885.
Ammoury N., Fessi H., Jean-Philippe Devissaguet J.-P., Allix M., Plotkine M., Boulu R.G., Effect on cerebral blood flow of orally administered indomethacin-loaded poly (isobutylcyanoacrylate) and poly (DL-lactide)nanocapsules. J. Pharm. Pharmacol. 42 558-561, 1990.
Rolland, A., Wagner, N., Chatelus, A., Shroot, B., Schaefer, H., 1993. Site-specific drug delivery to pilosebaceous structures using polymeric microspheres, Pharm. Res. 10 1738-1744.
de Jaron, E.G., Blanco-Prieto, M.J., Ygartua, P., Santoyo, S., 2001. PLGA microparticles: possible vehicles for topical drug delivery. Int. J. Pharm. 226, 181-184.
Corresponding Author: Raimar Löbenberg, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, T6G 2N8, Canada. rloebenberg@pharmacy.ualberta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps