J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(2):231-237, 2003
Therapeutic and hemolytic evaluation of in-situ liposomal preparation containing amphotericin - b complexed with different chemically modified b - cyclodextrins.
K.K. Chakraborty 1
Research and Development Division, Hindustan Antibiotics Ltd., Pimpri, Pune, IndiaS.R. Naik
Professor and Head of Pharmacology and Biotechnology, Principal K. M. Kundnani College of Pharmacy, Dr. Thadani Marg, Worli Sea Face, Mumbai, IndiaReceived 1 April 2003, Revised 25 May 2003, Accepted 16 June 2003
PDF version
Abstract
Purpose: The objective of this study was to evaluate therapeutic and haemolytic effects of liposomal preparation derived from proliposome entrapping inclusion complex of amphotericin B (AmB) with the chemically modified b-cyclodextrin (b-CD). METHODS: a series of liposomal AmB formulations with varying b-CD i.e. Hydroxy propyl b-CD (HPBCD) and Sulfo butyl ether b-CD (SBEBCD) having similar AmB content (0.5 mg/kg) were prepared and their effect compared with conventional liposomal amphotericin B (L-AmB) and free AmB on erythrocyte lysis and antifungals activity in experimental aspergillosis- and Cryptococcosis- mice model in-vivo . RESULTS: the liposomal AmB - HPBCD and AmB - SBEBCD found to be 6 times less toxic than free AmB or conventional liposomal AmB. Experimental findings indicate that infected animals treated with L-AmB entrapped inclusion complexes significantly reduced CFU values (fungal counts), whereas infected animals treated with conventional liposome or free AmB showed insignificant reduction in CFU. A marked increase in the percent survival was observed in the case of animals treated with liposomal AmB formulation (HPBCD/SBEBCD). Furthermore, the in-vitro toxicity (haemolysis) of the proliposome-based liposomal vesicles (PBLV) entrapped AmB-SBEBCD/HPBCD at 37°C was approx. 50% at maximum of the conventional liposomal AmB at a dose of 118 mg/ml as measured after 1 hr. incubation. CONCLUSIONS: the results of these experiments permitted us to conclude that the stabilization of liposome derived from proliposome entrapping inclusion complex of amphotericin B (AmB) with b-CD could serve an alternative approach to enhance the therapeutic window of AmB in clinical medicine.
Introduction
In the management of fulminant infections, the use of several lipid-based formulations of amphotericin-B: AmBisome (AmB-liposome), Abelcet (AmB-lipid complex), and Amphocil (AmB-colloidal dispersion) have been shown to reduce toxicity greatly, allowing higher doses to be given and thereby improving clinical efficacy (Storm and Crommelin et al, 1998). However, all of these formulations reported to date suffer from drawbacks in terms of stability (Washington et al 1993 & Kirsch et al 1998) and suitability for bulk manufacture for pharmaceutical applications. These vary from a requirement for pharmaceutically unacceptable solvents, which could give rise to undesirable solvent residues, to the requirement of sonication and extruders (Maitani et al., 2001). Therefore, A new hydroalcohalic method for the preparation of liposomes has been developed based on a proliposome preparation (Perrett et al 1991& Park et al., 1999) and ethanol injection (Maitani et al., 2001) entrapping inclusion complex amphotericin B (AmB) with different chemically modified b-cyclodextrins.
Previous work (Chakraborty & Naik, 1998) has already established the optimal conditions (in terms of structural organization of proliposome mixtures) for the entrapment of inclusion complexes (AmB-bCD) into vesicles and its stabilization in-vitro. Furthermore, we have also observed earlier that the inclusion complex (AmB - SBEBCD) protects the drug more efficiently against destructive interaction with the serum lipoproteins and cell membrane, in the cavity of this modified β-cyclodextrin (Chakraborty and Naik, 2001).
The present communication reports the comparative haemolytic and therapeutic efficacy of free AmB, solvent based conventional liposomal AmB, and proliposome based liposomes complexed with the varying b-cyclodextrin derivatives in in-vitro and in-vivo experiments.
Materials and Methods
Materials
Fungizone, a commercial deoxycholate preparation of amphotericin B, was obtained from Ambalal Sarabhai Enterprises, Baroda, India and was reconstituted to obtain the concentration 0.25g/L with 5% dextrose solution prior to use. Amphotericin B and L- phosphotidylcholine from egg yolk were purchased from Sigma Chemicals, Saint Louis, MO, USA. AmB was radiolabelled with 3H isotope (obtained from Bhabha Atomic Research Centre, Mumbai, India) using chloramine-T according to the procedure of Hunter (1978). Cholesterol was obtained from Centre for Biochemical (CSIR), Delhi, India. b-Cyclodextrin derivatives i.e. Hydroxypropyl b-CD (HPBCD) and Sulfo butyl ether b-CD (SBEBCD) were obtained from M/s. Cyclo Lab. Budapest, Hungary. All other reagents used were of analytical grade obtained locally. Male Balb/c mice weighing 20-25 g were obtained from National Institute of Nutrition (Hyderbad, India). Conventional-solvent based AmB-liposomes were prepared as described by Surolia et al (1975).
Preparation of Liposomes (Hydroalcoholic proliposome method)
Formulation of inclusion complex
The preparation involved complexation of amphotericin B (AmB) with different b-cyclodextrin derivatives (HPBCD/SBEBCD) by co-grinding method (Adhage & Vavia 1998). AmB and HPBCD or SBEBCD powders were mixed in the molar ratio of 1:2. This mixture was ground in a ball mill at a speed of 50 rpm for 6h and the complex formation was confirmed by molecular sieve chromatography of radiolabelled supernatant sample using a sephadex G-10 (Pharmacia) column. Whereas, the verification of the inclusion complex formation was with differential scanning calorimetry (Giron 1997) using a Perkin - Elmer DSC7 over a 100-250°C temperature range, scanning rate (10°C /min) and 5mg sample weight. Baseline calibration was performed prior to each run. The solid inclusion complex was dissolved in 0.15M Phosphate Buffer saline (PBS) pH 7.4, to solubilize inclusion complex. The drug b-CD suspensions were then centrifuged at 100000xg for 60 min. at 4°C to yield pellets and clear supernatants. Radioactivity measurements (LKB-1275 Minigamma meter Turku, Finland) showed that, most of the drug was solubilized in the supernatant (presumably in the form of inclusion complex), with a small amount of insoluble drug present in the pellet. The supernatants (0.5ml), which contained, 1mg AmB and 2.26 mg HPBCD or 1.9 mg SBEBCD derivative were used for entrapment into liposomes, manufactured from egg phosphotidyl chlorine (EPC) and cholesterol (Chol) by the proliposome method. In the formed inclusion complex, molar ratio of AmB: HPBCD was 1:1.8, whereas in case of AmB: SBEBCD, it was 1:1.4.
Entrapment of inclusion complex into liposomes
The preparation of liposomes derived from proliposome technique (Perrett et al 1991) with slight modification (Chakraborty & Naik 1998) was used to entrap inclusion complex (AmB- b-CD derivatives) into the aqueous phase of liposomes. In brief, proliposome mixture was prepared by transferring lipid containing EPC-Chol at molar ratio 7:1 into a round bottom flask. The lipid was thoroughly dried under nitrogen and dissolved in ethanol (10 ml) and then added PBS, pH 7.4 (20 ml) to yield a lipid-ethanol- water mixture (100:80:200). This mixture was heated to 60°C for few minutes and then allowed to cool to room temperature (25°C) yielding a proliposome mixture, which was finally converted into a multilamellar liposome (MLV) suspension by drop wise addition of PBS (pH 7.4) containing amphotericin-B complexed with different chemically modified b-cyclodextrin in the molar ratio of AmB/HPBCD (1:1.8) and AmB/SBEBCD (1:1.4) to a final volume of 10 ml. The suspension was vortex mixed throughout this last stage (Chakraborty & Naik 2000).
Non-entrapped complexes were separated from liposomes-entrapped complexes by diluting the MLV suspension with 0.15M PBS (pH 7.4) and centrifuged at 40000xg for 30 min. at 4°C. The pellets were washed with 5ml PBS and centrifuged again as above. The final liposomal pellets with entrapped inclusion complex were suspended in 1ml PBS. The appearance and the size distribution of the AmB - liposomes, prepared by conventional and proliposome method, were extensively discussed in our earlier publication (Chakraborty & Naik, 2000). Although both were multilayered, AmB liposomes prepared by proliposome method exhibited an average size between 3 - 5 mm, whereas liposome prepared by the conventional solvent - based method was around 6 - 8 mm.
Determination of Liposome intercalated AmB
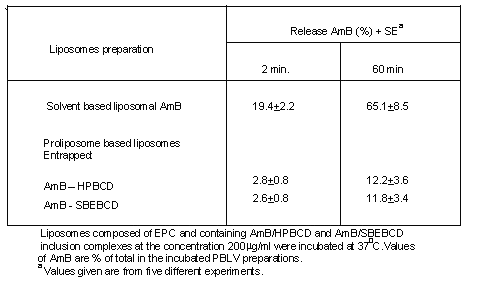
Phospholipid content was determined according to the procedure reported by Vaskovsky et. al. (1975). The amount of AmB intercalated into liposomes was determined by dissolving an aliquot of liposomal preparations in methanol and measuring the absorbance of AmB at 405 nm by uv-vis spectrophotometrically (Shimadzu, Japan) and subsequently confirmed by HPLC analysis (Nilsson - Ehle, et. al., 1977). AmB assay as a complex was estimated based on radioactivity used initially and radioactivity recovered in the washed MLV pellet for the presence of radioactive marker 3H using LKB-1275 Minigamma meter (Turku, Finland). Entrapment efficiency of AmB as an inclusion complex intercalated into liposomes (not shown) based on radioactivity measurements (Chakraborty & Naik 2000) were 37.4%+3.2 (AmB/HPBCD) and 45.1%+ 0.9 (AmB/SBEBCD).
In-vitro drug release studies
The stability of liposomes in terms of AmB loss was evaluated by the method of Juliano et.al. (1986). A suspension of liposomes containing 16wt percentage AmB at the concentration of 200mg AmB/ml in PBS (pH 7.4) was divided into several portions. One was immediately assayed for AmB contents, and other was incubated for varied periods at 37°C. Each sample was centrifuged at 15000 xg for 45 min, and the supernatant was removed. The remaining pellet was washed twice with PBS (pH 7.4) and then resuspended in fresh PBS (pH 7.4) so that the volume of the final suspension was the same as that of original volume. Finally, the amount of residual AmB was determined as described earlier.
In-vitro toxicity
The extent of haemolysis is an important parameter of toxicity of AmB to erythrocytes (Mehta et.al. 1989, Forster et.al 1998), while evaluating different liposomal AmB preparations. In brief, blood samples were drawn from Balb/c mice in the presence of heparin (50-units/ ml) and washed 3 times with 0.15M PBS (pH 7.4). A series of free AmB samples were prepared for haemolysis experiment by further diluting stock solution of AmB in PBS (pH 7.4) to get varying concentration ranged 0 - 50 mg AmB / ml, whereas dose levels of AmB used in the in-vitro analysis assay was ranged from 0 - 250 mg AmB /ml for PBLV. Subsequently, 0.8 ml of 0.1% red blood cells (vol. /vol.) were mixed with 0.2ml of buffer containing varying amounts of free AmB and proliposome-based liposomal vesicle (PBLV) entrapped inclusion complex. The dose levels of AmB used in the in-vitro haemolysis assay was ranged from 0 to 250 mg AmB/ml. The mixture was then incubated at 37°C for 1 hr and centrifuged at 1000xg for 2 min. The amount of haemoglobin released in the presence of 0.3% Triton X-100 was taken as measure of complete (100%) lysis.
Mouse model of cryptococcosis
Seventy-five male Balb/c mice (20 - 25g) (obtained from the National Institute of Virology Pune, India) were infected with 0.25ml Cryptococcosis neoformans cell suspension (7x10 6 cells ml-1 ) in normal saline via the caudal vein. The infected mice were divided into 5 groups. Group 1 received free AmB (0.5mg Kg-1 , I.V.); groups 2, 3 & 4 received solvent-based liposomal AmB and proliposome-based liposomal AmB - HPBCD & AmB - SBEBCD (0.5mg kg -1 I.V.) and group 5 received physiological saline (1ml kg-1). Each group (excepting 5) was administered with their respective amphotericin B preparation on an alternate days starting from the 3rd day of infection for 13 days, similarly group 5 was treated with saline as control. The progress of infection and mortality of mice were monitored for 13 - 16 days. Survival rate was recorded with the last dose of drugs. The mice were killed 2 days after the last dose and the fungal load was determined, in terms of colony forming units (CFU) in lung, liver, kidney, spleen and brain.
Mouse model of Aspergillosis
The aspergillosis animal model was established by the method described by Ahmad et.al (1989). 50 male Balb/c mice (20 - 25 g) were infected by administering intravenously 0.25ml Aspergillus fumigatus spore suspension 2x107 cell ml-1 in physiological saline via the caudal vein. After 2h, mice were divided into 5 groups of 10. The 1st and 2nd group of mice received liposomal AmB HPBCD/SBEBCD (0.5-mg kg-1 I.V.) and the 3rd group received solvent based liposomal AmB, whereas 4th group received free AmB (0.5-mg kg-1 I.V.). The 5th group (control) received physiological saline (1ml kg-1 ) and survival of mice for 7 days after the therapy was recorded. The CFU in lung, liver, kidney, spleen and brain was determined by sacrificing the mice on the 7th day after therapy.
CFU determination
The in-vivo antifungal activity was evaluated on the basis of survival rate of animals and number of CFUs in homogenates of lungs, liver, kidney, spleen and brain. The organs were excised aseptically washed with physiological saline and then homogenized in saline. A 25-fold serial dilution was placed in Sabouraud dextrose agar plates and then counting for CFU after 48h incubation at 37°C(Ahmad et al, 1989).
Statistical analysis
The CFU data were statistically evaluated by analysis of variance of one-way classification with unequal frequencies (Snedecor & Cochran 1968). The heterogeneity of means for the various organs were tested by the F ratio of treatment variance to the experimental error variance. The survival data were analyzed using Chi-squared with Yates correction and by Fisher's exact test (Fisher et al, 1981).
RESULTS
The stability of the liposomes was evaluated based on AmB release profiles. As shown in Table 1, higher retention of AmB within liposomes were observed when the AmB complexed with sulfobutyl ether (88.2%) and hydroxypropyl b-Cyclodextrin (87.8%) in comparison to conventional liposome (without b-CD) where it was (34.9%).
Table 1. Release of drug (AmB) from liposomes, prepared by using different b-CD derivatives.
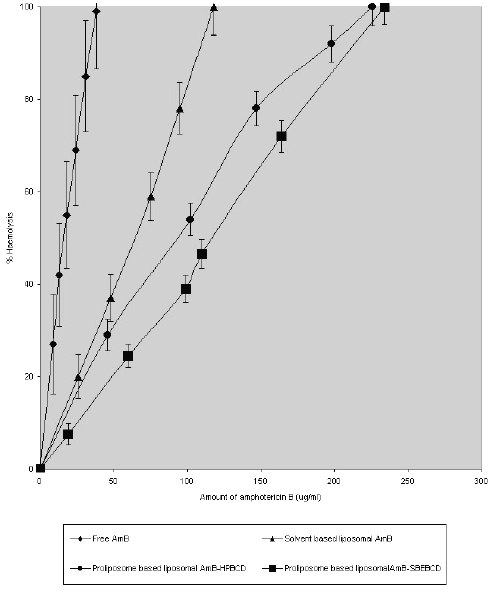
Fig. 1. shows the haemolytic ability of liposome encapsulated AmB-HPBCD and AmB-SBEBCD prepared by the proliposome method.
Figure 1: Erythrocyte lysis caused by amphotericin B when delivered through different liposomal preparations. Values are expressed as mean of % lysis of three separate experiments + standard error of means.
The dose level of AmB ranged from 0 to 250 mg AmB /ml. In all cases, the degree of haemolysis increased as the dose increased. However, at any dose of AmB, the haemolytic ability of the different liposomes showed considerable differences. For instance, liposome entrapped AmB - SBEBCD & AmB-HPBCD and solvent-based liposomal AmB showed comparative mild toxicity (15, 22 & 27% lysis of erythrocytes) at a concentration of 38 mg/ml, whereas at the same concentration of free AmB complete lysis (100%) of erythrocytes was observed. The corresponding haemolytic value (100% lysis) for AmB intercalated in solvent-based liposomes and proliposome-based liposomes entrapping AmB-HPBCD & AmB-SBEBCD complex was estimated to be 118, 226 and 234 mg /ml respectively.
Therapeutic efficacy of various liposomal AmB preparation on Cryptococcosis and Aspergillosis mouse model
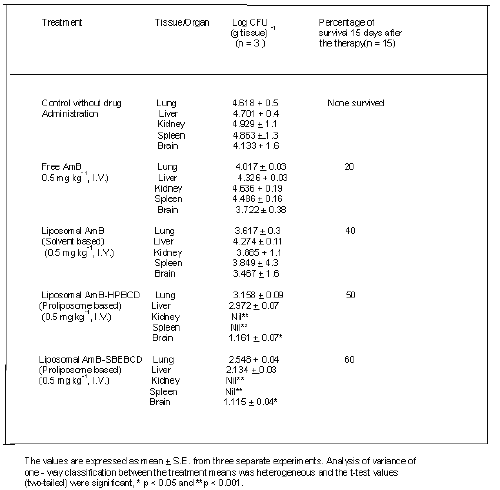
Cryptococcosis neoformans - infected mice treated with liposomal (AmB - HPBCD or AmB-SBEBCD) showed significant reduction in CFU values in lung, kidney and spleen compared with control animals (Table 2). The single dose (0.5-mg kg-1 I.V.) was administered to the in-vivo situation considering the degree of haemolysis (100 %) elicited by free AmB at a concentration of 38 mg /ml. The CFU reduction effect of liposome entrapped AmB - SBEBCD was higher than the liposome entrapped AmB - HPBCD. It was also observed that AmB -HPBCD and AmB -SBEBCD treated mice showed a complete absence of CFU in the spleen and kidney.
Table 2: Colony-forming units (CFU) of Cryptococcus neoformans in different organs and percent survival of infected mice and effect of chronic treatment with free and liposomal AmB.
Treatment with free AmB showed a significant reduction in CFU values in the lung only when compared with non-treated control mice. It was observed that control animals infected with Cryptococcosis neoformans elicited more than 50 and 90% mortality after 9 and 15 days respectively. Treatment with free AmB, conventional liposomal AmB and liposomal AmB-HPBCD/SBEBCD increased the survival rate by 20, 40, 50 and 60% respectively.
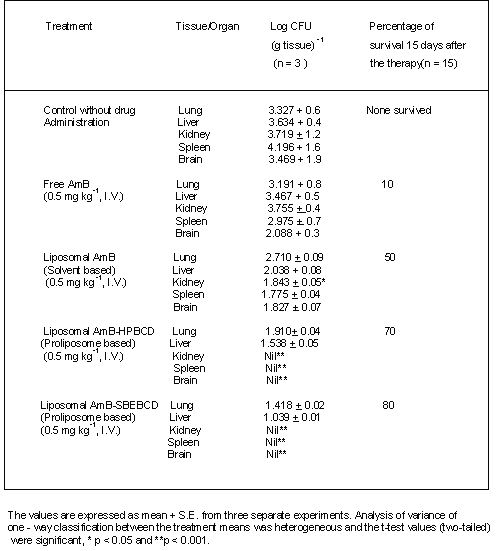
Aspergillosis mice treated with free AmB showed a significant reduction in CFU in both brain and spleen when compared to untreated control mice (Table 3). Infected animals receiving liposomal AmB - HPBCD and AmB - SBEBCD showed significant reductions in CFU in both lung and liver with no CFU being observed in brain, spleen or kidney (Table 3). All the control mice infected with A. fumigatus died within 5 to 7 days. Infected mice treated with free AmB, conventional liposomal AmB and liposomal AmB - HPBCD and AmB - SBEBCD showed 10, 50,70 and 80% survival respectively.
Table 3: Colony-forming units (CFU) of Aspergillus fumigatus in different organs and percent survival of infected mice and effect of chronic treatment with free and liposomal AmB.
DISCUSSION
The liposome derived from proliposome containing AmB displayed a distinctly decreased haemolytic activity of AmB as compared to that of free AmB or AmB in solvent based conventional liposomes.
It has been documented that mammalian cell (RBC) toxicity arises from the formation of conducting pores in the cell membrane, when AmB binds to cholesterol, a major sterol in mammalian membranes (Khutorsky, 1992; Hoogevest, et. al., 1978; De - Kruijiff et.al., 1974; and Bittman et. al., 1974). It is likely that inclusion of AmB - HPBCD & AmB - SBEBCD reduces the leakage of AmB from proliposome based liposome vesicles and thereby its directly interaction with red blood cells, leading to decreased lysis (McCormack and Gregoriadis, 1996). Our experimental findings clearly demonstrated that the PBLV entrapped AmB - SBEBCD inclusion complex were found to be more stable than the PBLV entrapped of AmB - HPBCD by their AmB release pattern. Which is in complete agreement with the findings of De-Chasteign (1996), suggesting that the longer the hydrophobic chain linked to the b-CD, the higher the association of drug within vesicle. However, it has also been demonstrated that the binding potential of the sulfobutylether b-CD derivative is not only dependent on both sulfobutyl chain length and degree of substitution, but also on the substrate (phospholipid) properties (Zia et. al., 1995). The binding constants for neutral forms of the drug (AmB) were always greater with SBEBCD than with HPBCD.
In the present investigation of antifungal activity (in-vivo) of amphotericin B, no significant differences were observed between the liposomal formulations of entrapped AmB-HPBCD and AmB-SBEBCD in the mouse models of aspergillosis and cryptococcosis. Our observations are in complete agreement with the earlier reports (Moonish et. al., 1993, Ahmad, et. al., 1989). In addition our observations also suggest the possibility of PBLV comprised inclusion complex (AmB - HPBCD / SBEBCD) behaving as a depot from which the free AmB is released slowly to the site of infection, which may be due to mechanical trapping of liposomes in the damaged alveolar capillaries (Moonish et al., 1993) and delivery of phagocytosed liposomes containing AmB to the infected site via circulating monocytes and/or macrophages. In addition, proliposome based liposomal AmB markedly slow down the rate of transfer of the amphotericinB from the vesicles to erythrocytes & other tissues and its subsequent removal from plasma, resulting in higher in-vivo stability, residence time and intrinsic permeability of drug - sterol complex (Chakraborty & Naik, 2001). Obviously, this specific physiological event might help to achieve a significant improvement in the therapeutic index of PBLV.
The other possible mechanisms by which PBLV offers protection against A.fumigatus and C.neoformans infection is the processes of selective uptake by macrophages, which appears to be a slow complex dissociation rather than rate of vesicle disintegration and largely account for the most of the drug metabolism in the tissues of Reticulo endothelial system (RES).
In conclusion, this is a simple and robust hydroalcohalic method for in-situ liposomal preparation that avoids pharmaceutically unaccepted solvents & energy expensive procedures such as sonication, suitable to use for bulk production in pharmaceutical industries. The use of co-grinding method in ball mill and subsequently controlled precipitation technique permitted the inclusion complex formation of tremendous cost effective and revealed a slower complex dissociation of lipophillic drug AmB from anionic SBEBCD than neutral HPBCD inclusion. The proliposome based liposomal vesicles containing AmB - HPBCD and SBEBCD prepared from structure organization of proliposome mixture in the molar ratio of lipid: ethanol: water (100:80:200) exhibited superior entrapment efficiency and minimal drug leakage as compare to conventional solvent based liposomal vesicles.
The present experimental findings clearly demonstrated the potential usefulness of proliposome-based liposome vesicles in improving therapeutic efficacy of AmB in the treatment of experimental systemic fungal infection by reducing haemolytic activity and also improving in-vivo antifungal activity. The proliposome approach of incorporating chemically modified b-cyclodextrins showed high binding affinity and specificity to accommodate water insoluble molecules (guest) in hydrophobic milieu of the cavity present in the modified b-cyclodextrins structure and circumvented liposomal stability problem especially in biological environment.
It is also possible that this approach to stabilization of liposomal preparation via drug/b-cyclodextrin inclusion complexes can also be extended to other wide range of chemotherapeutic agent's viz. immuno suppressants and anticancer agents etc. to enhance and improve their therapeutic window and reduce their toxicity profile.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the valuable advice and constructive criticism during research work by Dr. S.K.Banerjee (Retd.) Professor, Dept.of Pharmacy, M.S. University of Baroda, India.
REFERENCES
Adhage, N.A., Vavia, P.R. Grinding: A method for b-cyclodextrin inclusion complexation, 17th Asian Congress of Pharmaceutical Sciences, 10-13 December, Mumbai (Bombay) India. PP: 58, 1998.
Ahmad, I., Sarkar, A.K., and Bachhawat, B.K. Liposomal amphotericinB in the control of experimental aspergillosis in mice: Part I- Relative therapeutic efficacy of free and liposomal amphotericinB. Indian Journal of Biochemistry & Biophysics. 26: 351-356, 1989.
Bittman, R., Chen, W.C., Anderson, O. R. Interaction of Filipin lll and amphotericin B with lecithin - sterol vesicles and cellular membrane - spectral and electron microscope studies. Biochemistry. 13: 1364-1373, 1974.
Chakraborty, K.K. and Naik, S.R. Pharmacokinetic analysis of In-Situ liposomal preparation derived from proliposome. Symposium on Natural origin substances in drug formulation, 4 to 6 November, Beijing China. PP: 49-50, 1998.
Chakraborty, K.K. and Naik, S.R. In-situ liposomal preparation containing amphotericin - B and its related toxicity and tissue disposition studies, Pharmaceutical development and Technology. 5(4): 543-553, 2000.
Chakraborty, K.K. and Naik, S.R. Pharmacokinetic studies of In-Situ liposomal preparation containing amphotericin B complexed with different chemically modified b-cyclodextrins. Journal of Liposome Research.11 (1): 1-14, 2001.
De - Kruijff, B., Demel, R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma Ladlawii cells and lecithin liposomes. ll. Temperature dependence of the polyene - sterol complex formation. Biochim. Biophys. Acta.339: 44-56, 1974.
De-Chasteign, S., Fessi, H., Davissaguet, J. P. and Puisieusx, F. Comparative study of the association of Itraconazole with colloidal drug carrier, Drug Dev. Res.38 (2): 125 – 133, 1996.
Fisher, B. D., D–Armstrong, B. Vn., Gold, J. W. M. invasive aspergillosis progress in early diagnosis and treatment. Am. J. Med., 71: 571, 1981.
Forster, D., Washington, C., Davis, S.S. Toxicity of solubilized and colloidal amphotericin B formulation to human erythrocytes. J. Pharm. And Pharmacol. 40:325 – 328, 1988.
Giron, D. Thermal analysis of drugs and products, Encyclopedia of Pharmaceutical Technology. (Swarbic, J., Boylan J.C. Ed.) Marcel Dekker Inc. New York. 15: 56-58, 1997.
Hunter, W.M. Handbook of experimental Immunology, Eds. Blackwell Sci., Oxford, 1978.
Hoogevest, P. V., Kruijiff B.D. Effect of AmphotericinB on cholesterol -containing liposomes of egg phosphotidylcholine and didocosenoyl phosphotidyl-choline- A refinement of the model for the formation of pores by amphotericin B in membranes. Biochim. Biophys. Acta. 511: 397-407, 1978,Hunter, W.M. (1978) In Handbook of experimental Immunology, Eds. Blackwell Sci., Oxford.
Juliono, R.L., Grant, C.M.W., Barber, K.R. and Kalp, A. Mechanism of selective toxicity of amphotericin B incorporated into liposomes.Mol.Pharmacol.31: 1-11, 1986.
Khutorsky, V. E. Structure of Amphotericin-B-cholesterol complex. Biochim Biophys. Acta. 1108:123-127, 1992.
Kirsch, R., Goldstein, R., Tarloff, J., Parris, D., Hook, J., Hanna, N. An emulsion formulation of amphotericin-B improves the therapeutic index when treating systemic murine candidiasis. Journal of Infectious Diseases. 158:1065-70, 1998.
Maitani, Y., Soeda, H., Junping, W., Takayama, K. Modified ethanol injection method for liposome containing b-sitosterol b-D- glucoside. Journal of Liposome Research.11 (1): 115-125, 2001.
McCormack, B., Gregoriadis, B. Comparative studies of the fate of free and liposome - entrapped hydroxypropyl - b - cyclodextrin/drug complexes after intravenous injection into rats: implications in drug delivery. Biochimica at Biophysica Acta. 1291: 237-244, 1996.
Mehta, R.T., Hopfer, R. L., Juliano, R. L., Lopez- Berestein, G. A comparison of in-vitro toxicity and antifungal efficacy of membrane - active drugs after liposome encapsulation. Selective Cancer Therapeutics. 5: 113 – 117, 1989.
Moonish, M., Ahmad, I., Bachhawat, B.K. Mannosylated liposomes as carriers for hamycin in the treatment of experimental aspergillus in Balb/c mice.J.Drug Targeting. 1:147-155, 1993.
Nilsson – Ehle, I., Yoshikawa, T.T., Edwards, J. E., Schotzand, M.C. and Guze, L. B. Quantitation of amphotericin B with use of high-pressure liquid chromatography, J. Infect. Dis., 135,414 – 422,1977.
Park, K., Lee, M., Hwang, K., Kim, C. Phospholipid based microemulsions of flurbiprofen by the spontaneous emulsification process. Int. J. Pharm. 183:145 – 154, 1999.
Perrett, S., Golding, M., Williams, and W.P. A simple method for the preparation of liposomes for pharmaceutical applications: Characterization of the liposomes. J. Pharm. Pharmacol. 43: 154-161, 1991.
Snedecor, G.W., Cochran, W.G. statistical method, Oxrord, & IBH Publ. Co. India. PP 227 –279, 1968.
Storm, G. and Crommelin, J.A. Liposomes: quo vadis? PSTT.1: 19 – 31, 1998.
Surolia, A., Bachhawat, B. K., Podder, S. K. interaction between lectin from ricinus communis and liposomes containing gangliosides. Nature. 257: 802–804, 1975.
Vaskovsky, V. E., Kostetsky, E. Y., Vasendin, I. M., A Universal reagent for phospholipid analysis. J. Chromatogr. 114: 129–141, 1975.
Washington, C., Lance, M., Davis, S. S. Toxicity of amphotericin B emulsion formulations. Journal of Antimicrobial Chemotherapy. 31:806-808,1993.
Zia, V., Bornanchini, E. R., Luna, E .A., Rajeswaki, R.A., Stella, V.J. Sulfoalkyl ether BCDS: Effects of alkyl chain length and degree of substitution on complexation, AAPS, Tenth Annual meeting and exposition, November 5-9, Miami Beach, Florida, PP: 127, 1995.ospholipid analysis. J. Chromatogr. 114: 129–141, 1995.
Corresponding Author: K.K. Chakraborty, Research & Development Division, Hindustan Antibiotics Ltd., Pimpri, Pune - 411 018, India. kkchakrabor@yahoo.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps