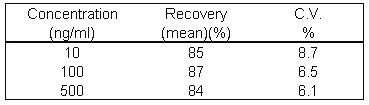J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(2):246-251, 2003
Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay.
Spencer Ling and Fakhreddin Jamali1
Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, CanadaReceived 04 March 2003, Revised 31 July 2003, Accepted 05 August 2003
PDF version
Abstract
PURPOSE. Stress increases plasma corticosterone concentration in rats. We studied the time-course of plasma corticosterone post jugular vein cannulation, a potential stress producing process, and after 1 h restraint stress using an improved assay. Current HPLC methods for corticosterone analysis require long extraction times with large volumes of solvent. METHODS. Adult male, Sprague-Dawley rats were used. Tail vein blood samples were collected from uncannulated rats (n=4). Other rats were cannulated in the right jugular vein, allowed to recover overnight and assigned to days 1, 2, 3 or 4 groups (n=4-6/group). Blood was sampled before and after exposure to 1 h of restraint stress. Plasma was separated and extracted with 5 mL ethyl acetate (betamethasone as internal standard), and then the extract was washed with sodium hydroxide (0.1 M) and water. After evaporation of ethyl acetate, the residue was dissolved in mobile phase (acetonitrile-water-acetic acid-TEA, 22:78:0.1:0.03, v/v) and injected into an isocratic HPLC consisting of a 10 cm C18 column, and UV detector at 254 nm. RESULTS. The minimum quantifiable concentration was 10 ng/mL of corticosterone based on 0.5 mL plasma. The standard curve was linear over the concentration range of 10-500 ng/mL. The extraction efficiency was 84-87%. The coefficient of variation for intra-day and inter-day precision was <10% with <8% error. Plasma corticosterone before cannulation (74 ± 17 ng/mL) and those measured daily before restraint stress were not significantly different from one day to another (day 1, 60 ± 21; day 2, 59 ± 39, day 3, 45 ± 23, and day 4, 41 ± 8 ng/mL). Exposure to restraint stress elevated corticosterone (day 1, 122 ± 33; day 2, 82 ± 23; day 3, 148 ± 33; and day 4, 134 ± 20 ng/mL). Except on day 2, all concentrations were significantly elevated as compared to the pre-stress levels. CONCLUSIONS. Corticosterone concentrations were stable after jugular vein cannulation, and were increased by restraint stress. The present assay is a more convenient method as compared to those previously reported.
Introduction
Blood vessel cannulation allows repeated blood sampling from conscious, unrestrained rats while avoiding the stress due to handling, restraint and anesthesia (1-3). The process, and the presence of the catheter itself, however, potentially induces stress. Cannulation surgery and physiological changes that occur because of a chronic catheter may evoke a stress response as indicated by a rise in plasma corticosterone. Surgery may cause changes in hormonal and hemodynamic responses (4) that are reduced when pre-surgery analgesics are given to decrease stress and pain (5). Catheter infection, while uncommon in rats, is known to stimulate a stress response, and is a potential consequence of long-term catheterization (6, 7). While an adequate recovery time from surgery (8) and aseptic surgical technique may minimize these effects, the precise time for full recovery is uncertain. Fagin et al. (8) showed that while plasma corticosterone levels stabilized three to four days after cannulation, they remained elevated compared with uncannulated rats. This is not surprising since recovery of the hypothalamic-pituitary-adrenal axis to acute stress may be prolonged following a severe stressor (9, 10). An adequate recovery time is therefore required to minimize the effects of surgery on a subsequent stressor (8, 10).
It would be advantageous to use a cannula within days of insertion. Besides the risk of infection in a chronic catheter, and the challenge of keeping the cannula patent for more than a week (1), other physiologic changes (e.g. altered levels of plasma proteins) have been observed (11). While a 7-day recovery period from surgery allowed rats to respond to experimentally induced stress (8), it is unclear whether a robust stress response could be detected 1 or 2 days following cannulation. To assess the optimum time to recovery from the stress of cannulation surgery, we studied the time-course of plasma corticosterone during recovery of the hypothalamic-pituitary-adrenal axis from jugular vein cannulation and the subsequent stress response.
High performance liquid chromatography (HPLC) methods for corticosterone analysis have been developed that are specific and sensitive (12, 13). They, however, require long extraction times with large volumes of solvent. We report the development of a reversed-phase HPLC method with a simple extraction method for the determination of corticosterone in rat plasma.
Materials and Methods
Chemicals
Corticosterone and betamethasone were obtained from Sigma (St. Louis, MO, USA). Acetonitrile (HPLC grade) and ethyl acetate (reagent grade) were obtained from Caledon Laboratories Ltd. (Georgetown, Canada). Decolorizing charcoal (Norit A) was obtained from BDH Inc. (Toronto, ON, Canada).
Apparatus and chromatographic conditions
The HPLC apparatus consisted of a Model 590 pump, a 712 WISP autosampler (Waters, Mississauga, ON, Canada), a 10 cm x 4.6 mm I.D. C18 analytical column packed with 5-mm particles (Phenomenex, Torrance, CA, USA), and a HPLC pre-column insert packed with C8 (Waters, MA, USA) particles. The detector was an SPD-10A ultraviolet visible detector (Shimadzu, Tokyo, Japan) set at 254 nm, and the recorder-integrator was a Model 3390A (Hewlett-Packard, Palo Alto, CA, USA). The mobile phase was acetonitrile-water-acetic acid-TEA (22:78:0.1:0.03, v/v), and was pumped at a flow-rate of 1.0 mL/min at ambient temperature.
Standard solutions
Stock solutions of corticosterone (2.5 mg/mL) and betamethasone (1.25 mg/mL) were prepared in ethyl acetate and stored at 4°C. Standard curves were prepared using pooled plasma treated with decolorizing charcoal to remove endogenous corticosterone (12,14,15). Decolorizing charcoal (0.05 g/mL of plasma) was added to rat plasma and stirred for 90 minutes at room temperature. The suspension was then centrifuged at 13 600 g for 8 minutes at 4-6°C. The plasma layer was decanted and filtered through a 0.45 mm Lida filter to remove carbon particles.
Sample preparation
To each 500 mL plasma sample was added 50 mL of internal standard (betamethasone, 1.25 mg/mL). This mixture was extracted with 5.0 mL of ethyl acetate, vortex-mixed for 30 seconds, followed by centrifugation at 1800 g for 10 min. The organic layer was transferred to clean tubes, then washed with 1.0 mL of 0.1 M NaOH and then with 1.0 mL of water, and centrifuged for 5 minutes. The organic layer was transferred to clean tubes, and evaporated to dryness. The residue was reconstituted in 170 mL of mobile phase and 150 mL was injected into the HPLC system.
Extraction efficiency
To determine the extraction efficiency of corticosterone from plasma, 0.5 mL of plasma was spiked with corticosterone at concentrations of 10, 100, and 500 ng/mL and extracted as described above. Unextracted standards consisted of corticosterone solutions prepared at the same concentrations, evaporated to dryness, then reconstituted with mobile phase, and injected into the HPLC system. Percentage recovery was calculated as follows:
Recovery (%) = (peak area of extracted plasma/peak area of unextracted standard)*100
Accuracy and precision
Plasma samples spiked with corticosterone at concentrations of 10, 25, 50, 100, 250, and 500 ng/mL were analyzed according to the procedure described above. Precision was determined by the calculation of inter- and intra-day co-efficients of variation (% C.V.). Accuracy was calculated as percentage error:
% error = (observed concentration - expected concentration)/expected concentration *100
Animal study
This study was approved by the University of Alberta animal ethics committee. Male, Sprague-Dawley rats (n=24) weighing between 250 and 300 g (Health Sciences Laboratory Animal Services, University of Alberta, Edmonton, Canada) were housed in the study area 24 hours prior to cannulation surgery, with a 12 h light/dark cycle (lights on at 8:00 a.m.). Under pentobarbital sodium 65 mg/kg i.p. (Somnotol; MTC Pharmaceuticals, Cambridge, ON, Canada) anesthesia, polyethylene (PE-50; Clay Adams, Parsippany, NY, USA) cannulas tipped with 2 cm of silastic (Dow Corning Corp, Midland, MI, USA) tubing, were inserted into the right jugular vein and exteriorized by subcutaneous tunneling to an incision made in the interscapular area. Rats were then allowed to recover overnight and had free access to food and water. Rats were divided into 4 groups and were exposed to stress on either 1, 2, 3 or 4 days (day 1, 2, 3, and 4) following surgery. On the day of experiment, 1.0 mL of blood was sampled immediately before (baseline) and after (stress) exposure to 1 hour of restraint stress to determine levels of corticosterone at baseline and in response to stress, respectively. Corticosterone levels of control (intact) rats (n=4) were measured by withdrawing blood from the tail vein of intact rats under halothane/O2 anesthesia. All blood samples were drawn between the hours of 8:00 and 11:00 a.m. After collection of blood samples, the plasma layer was separated by centrifugation and stored at -20°C until analysis.
Statistical Analysis
The significance of the observed differences between the control (intact) and baseline (cannulated) corticosterone was assessed using the one-way ANOVA followed by the Duncan's New Multiple test. For cannulated rats, the difference between baseline values and those after stress was tested using the paired Student's t-test. For all tests a was at 0.05.
Results and discussion
Corticosterone Assay
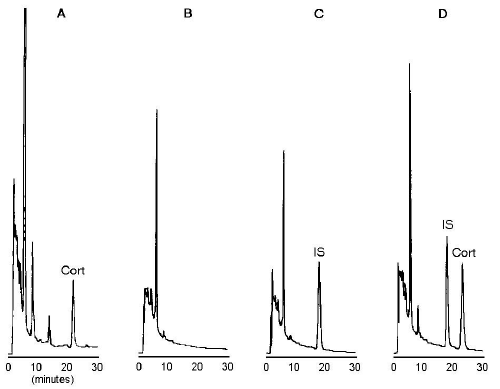
Representative chromatograms of untreated plasma, charcoal-treated plasma, and charcoal-treated plasma spiked with internal standard and 100 ng/mL of corticosterone are shown in Figure 1.
Figure 1: Chromatograms of untreated plasma containing endogenous corticosterone (Cort) (A), charcoal-treated plasma (B), charcoal-treated plasma spiked with internal standard (betamethasone) (C), and charcoal-treated plasma spiked with internal standard and corticosterone (100 ng/mL) (D).
Endogenous corticosterone was present in untreated plasma, with a peak retention time of 22.3 minutes (Figure 1A).
No interfering peaks were observed in the charcoal-treated plasma, at the retention time of corticosterone or internal standard (Figure 1B).
The retention time was 17.3 minutes for the internal standard peak (Figure 1C), and 22.5 minutes for corticosterone (Figure 1D).
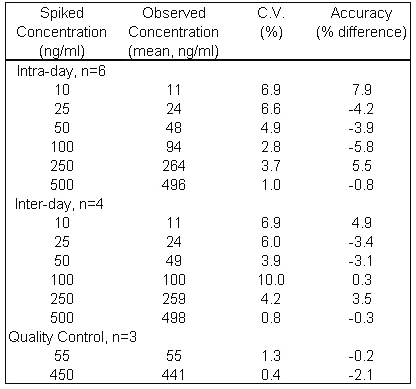
Standard curves showed excellent linearity (r > 0.99) between the peak-area ratios of corticosterone:internal standard and corticosterone concentration. A typical standard curve could be described by the equation, y = 0.0095x - 0.0022 over a range of 10 - 500 ng/mL. As shown in Table 1, accuracy of the assay was within 7.9% of the spiked standards and the intra- and inter-day coefficient of variation (C.V.) of the assay ranged from 1.0 to 6.9% and 0.8 to 10%, respectively.
Table 1: Intra- and inter-day variability of assay of corticosterone spiked in rat plasma.
Extraction efficiency was at least 84% at each of the concentrations as shown in Table 2
Table 2: Extraction efficiency of the assay for corticosterone spiked in rat plasma (n=3).
Animal Study
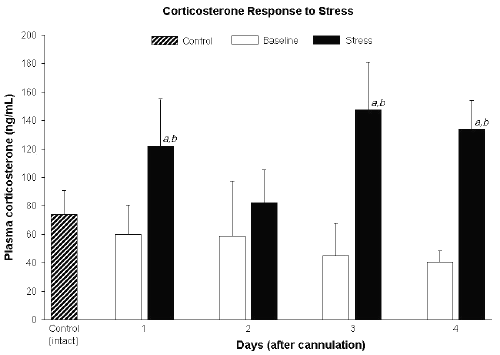
Plasma corticosterone measured daily before restraint stress (baseline) were not significantly different from one day to another and were similar to corticosterone levels in the control (intact) group (Figure 2).
Figure 2: Plasma corticosterone in control (intact) rats and those before (baseline) and after 1 h restraint stress in cannulated rats. Data expressed as mean ± SD.
a Significantly different from control (p<0.05).
b Significantly different from baseline (p<0.05).
Exposure to restraint stress significantly elevated corticosterone concentrations compared to the pre-stress levels, except on day 2 (Figure 2).
Discussion
We describe an HPLC method that is accurate and precise and suitable for determination of corticosterone in rat plasma. We used an established method, i.e., removing endogenous corticosterone by carbon treatment, for construction of our standard curves (12,14,15). The extraction procedure was simple and rapid. A smaller volume of solvent was required, 5 mL ethyl acetate, compared with previous assay (12), which required 15 mL of methylene chloride. This extraction required only 30 seconds of vortex mixing compared with 15 minutes on a shaker (12). This yielded good extraction efficiency, at least 84% at all concentrations, and very good accuracy and precision; accuracy within 7.9% of the spiked standards and the intra- and inter-day coefficients of variation of the assay ranged from 1.0 to 6.9% and 0.8 to 10%, respectively. The drug and internal standard were resolved with no interfering peaks. The peak elution times of 17 and 22 min were observed for internal standard and corticosterone, respectively, which were longer than 9 and 12 min reported previously (12). This is due likely to our use of a more polar mobile phase.
Our results show that baseline plasma corticosterone levels in jugular vein cannulated rats were not significantly different from basal corticosterone in uncannulated rats. These levels were similar to literature reports of corticosterone levels in jugular vein cannulated rats (16) and carotid artery cannulated rats after they had returned to baseline (8). Whereas Fagin et al. (8), observed plasma corticosterone to be elevated initially after cannulation and then declining by day 3, we observed stable corticosterone levels for four days following surgery. This may be due to an inherent difference between jugular vein and carotid artery cannulation. Carotid cannulation is a more stressful procedure than jugular cannulation, and can cause marked weight loss as well as sympathetic nerve damage (1).
Cannulated rats responded to 1 hour of restraint stress with elevated plasma corticosterone concentrations on all four days following surgery, although the rise was not significant on day 2. The lack of significance in corticosterone concentration on day 2 may be attributed to the high relative variability in the data on that day (Figure 2). Repeated blood sampling has been shown to increase plasma corticosterone levels, particularly in the first 0.5 h (17), and could possibly have contributed to the variability in our data. The effect of sampling time however, was likely minimal, as Vachon and Moreau (2001) demonstrated that the effect of sampling on corticosterone level is no longer apparent by 1 h following the first sampling time. Our post-stress blood samples were collected at least 1 h following the first blood sample. Furthermore, the rise in corticosterone levels in our study are a good reflection of the effects of restraint stress since peak corticosterone levels have been shown to occur at 1 h following 1 h of restraint stress (10).
In the case of a more intense stressor such as carotid artery cannulation, one would expect a less obvious rise in corticosterone level in response to stress due to the already elevated levels of corticosterone. In addition, it has been suggested that rats previously exposed to stress may exhibit a reduced response to a subsequent stressor due to a combination of negative feedback by corticosterone, and adaptation of the HPA axis (18,19). Our data however showed a robust corticosterone response to stress following surgery. This may be explained by evidence that the HPA axis is unlikely to show adaptation to a stressor that is different from the previous one (20).
In summary, our data suggest that jugular vein cannulation does not alter subsequent stress responses and therefore may be used within days of the surgical procedure without the confounding effects of stress. The HPLC assay presented here is sensitive, reliable and simple. It has been used to assess plasma corticosterone levels in the rat following jugular vein cannulation, and subsequent response to stress.
Acknowledgements
This work was supported by a grant 983587 of the Canadian Institutes of Hearth Research. SL is a recipient of Rx&D-HRF/CIHR Graduate Research Scholarship.
References
Yoburn, BC., Morales, R., Inturrisi CE. Chronic vascular catheterization in the rat: comparison of thee techniques. Physiol Behav, 33:89-94, 1984.
Suzuki, K., Koizumi, N., Hirose, H., Hokao, R., Takemura, N., Motoyoshi, S. Blood sampling technique for measurement of plasma arginine vasopressin concentration in conscious and unrestrained rats. Lab Anim Sci, 47:190-3, 1997.
Reilly, J. Variables in animal based research: Part 2. Variability associated with experimental conditions and techniques. ANZCCART News, 11:1-12, 1998.
Weissman, C. The metabolic response to stress: an overview and update. Anesthesiology, 73:308-327, 1990.
George, JM., Reier, CE., Lanese, RR., Rower, JM. Morphine anaesthesia blocks cortisol and growth hormone response to surgical stress in humans. J Clin Endocrinol Metab, 38:736-741, 1974.
Popp, MB. and Brennan, MF. Long-term vascular access in the rat: importance of asepsis. Am J Physiol, 241:H606-12, 1981.
Bradfield, JF., Schachtman, TR., McLaughlin, RM., Steffen, EK. Behavioral and physiologic effects of inapparent wound infection in rats. Lab Anim Sci, 42:572-8, 1992.
Fagin, KD., Shinsako, J., Dallman, MF. Effects of housing and chronic cannulation on plasma ACTH and corticosterone in the rat. Am. J. Physiol, 245 (Endocrinol. Metab 8):E515-E520, 1983.
Fleshner, M., Deak, T., Spencer, RL., Laudenslager, ML., Watkins, LR., Maier, SF. A long term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology, 136:5336-5342, 1995.
Garcia, A., Marti, O., Valles, A., Dal-Zotto, S., Armario, A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Neuroendocrinology, 72:114-125, 2000.
Terao, N. and Shen, DD. Alterations in serum protein binding and pharmacokinetics of l-propranolol in the rat elicited by the presence of an indwelling venous catheter. J Pharmacol Exp Ther, 227:369-75, 1983.
Wong, YN., Chien, BM., D’mello, AP. Analysis of corticosterone in rat plasma by high-performance liquid chromatography. J Chromatogr B, 661:211-218, 1994.
Woodward, C. and Emery, P. Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr, 419:280-284, 1987.
Jusko, WJ., Pyszczynski, NA., Bushway, MS., D’Ambrosio, R., Mis, SM. Fifteen years of operation of a high-performance liquid chromatographic assay for prednisolone, cortisol and prednisone in plasma. J Chromatogr B, 658:47-54, 1994.
Doppenschmitt, SA., Scheidel, B., Harrison, F., Surmann, JP. Simultaneous determination of prednisolone, prednisolone acetate and hydrocortisone in human serum by high-performance liquid chromatography. J Chromatogr B, 674:237-246, 1995.
Phelps, CP., Chen, LT., Oliver, J., Poole, LL., Menzies, RA. Variable tissue reactions and endocrine responses to a jugular catheter. J Submicrosc Cytol Pathol, 27:83-89, 1995.
Vachon, P., Moreau, JP. Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. Contemp Top Lab Animal Sci, 40(5):22-24, 2001.
Rivier, C. and Vale, W. Diminished responsiveness of the hypothalamic-pituitary-adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinology, 121:1320-8, 1987.
Marti, O., Andres, R., Armario, A. Defective ACTH response to stress in previously stressed rats: dependence on glucocorticoid status. Am J Physiol, 277(3 Pt 2):R869-77, 1999.
Armario, A., Hidalgo, J., Giralt, M. Evidence that the pituitary-adrenal axis does not cross-adapt to stressors: comparison to other physiological variables. Neuroendocrinology, 47:263-7, 1988.
Corresponding Author: Fakhreddin Jamali, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, T6G 2N8, Canada. fjamali@pharmacy.ualberta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps