J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(1):95-100, 2003
Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells.
Zhaolin Wang*, H.T. Chen**, Wilson Roa**, Warren Finlay*1
*Aerosol Research Laboratory, Department of Mechanical Engineering, **Cross Cancer Institute, Department of Oncology, University of Alberta, Edmonton, Alberta, CanadaReceived 30 January 2003, Revised 17 April 2003, Accepted 28 April 2003
PDF version
Abstract
PURPOSE: A nebulized aerosol formulation of the anti-cancer agent farnesol is developed and shown to induce cell death of human lung cancer cells in vitro. METHODS: A nebulized farnesol formulation containing polysorbate 80 (Tween 80) is developed. The measurements of the aerosol properties during nebulization were used as input for a mathematical model of airway surface liquid in the lung of an average adult, to estimate the airway surface liquid drug concentration of the deposited farnesol. Cytotoxicity of the formulations was measured in vitro on non-small cell lung cancer cells (H460 and A549). RESULTS: As much as 100% of lung cancer cytotoxicity can be achieved by using Pari LC Star and LC Plus nebulizers. The estimated airway surface liquid concentrations of the deposited farnesol reveal that the IC50 of the nebulized farnesol can be achieved over the entire tracheobronchial region, using the above Pari nebulizers with a volume fill of 5 ml. CONCLUSIONS: Drug concentrations higher than IC50 in the airway surface liquid are predicted with our methods, suggesting in vivo trials of a formulation may be warranted with these particular nebulizers.
Introduction
Conventional approaches to lung cancer treatment, including traditional chemotherapy, have shown relatively limited success (1). Indeed, the overall 5-year survival rate of 14% has not changed for 50 years (1-2). Thus, it is clear that new or combinational therapies that create maximum anti-tumor response without damage of normal respiratory tract tissues are needed.
In this regard, Burke et al. published a seminal paper describing inhibition of cancer growth by dietary farnesol and geraniol (3). This opened the door to clinical trials with other isoprenoids, including the monoterpenes limonene and perillyl alcohol, on cancer patients (4-6). It also initiated development of laboratory research on the mechanism of action of isoprenoids in cancer prevention and cancer cells (7-10).
Recent advances in technology have also created renewed interest in the inhalation delivery of drugs, including those for the treatment of lung cancer (11-12). Regional drug delivery via inhalation offers many advantages in the administration of pharmaceutical compounds for the prevention and treatment of respiratory diseases because the drugs are delivered at intensified dose levels directly to the site of disease, limiting systemic exposure.
In view of the above success with farnesol and the potential ability of inhaled aerosol delivery, the authors embarked on a program to test nebulized farnesol for inhalation delivery.
Materials and Methods
Trans, trans-farnesol (i.e. E, E-farnesol) is a sesquiterpene used in perfumery. It is not water soluble, and is a mild irritant, not classified as hazardous by the EPA and `Worksafe' Australia. Researchers have shown that farnesol causes apoptosis of A549 lung carcinoma cells (13-14). The reported IC50 of farnesol for human lung adenocarcinoma A549 cells is in the mM range. Because of its insolubility in water, formulating farnesol at these concentrations for nebulization requires special considerations. Formulating farnesol with phospholipids is not the first choice since phosphatidylcholine and/or diacylglycerol inhibit the activity of farnesol and geranylgeraniol (15). Instead, formulation with a surfactant was investigated as follows.
Materials
Trans, trans-farnesol and polysorbate 80 (Tween 80) were purchased from Aldrich Chem. Co. (Milw, WI, USA). Anhydrous ethyl alcohol (USP) was purchased from Commercial Alcohol Inc. (Brampton, Ontario, Canada). Methanol (HPLC grade) was from Fisher Scientific, Canada. Respigard bacterial/viral #303 filters were purchased from Marquest Medical Products Inc (Englewood, CO, USA). Cell proliferating Kit II (XTT) was purchased from Roche Molecular Biochemicals (Laval, Quebec, Canada). All materials were used as received.
Formulation
Trans, trans-farnesol is a polyunsaturated sesquiterpene alcohol, lighter than water and immiscible with water. It has a molecular weight of 222.37 g/mol. Commercially available farnesol is 96% pure, UV lmax =192-196 nm (for farnesol) and 238 nm (for impurities, e.g. E, E-farnesene or other compounds containing conjugated double bonds).
Ingredients : Farnesol 60 mL (i.e. 52.74 mg), 56.8 ml of Tween 80 44.2 mg/ml ethanol (i.e. 2.51 mg), ethanol 943.2 ml were used, with 0.9% aqueous NaCl added to give 5 ml. The first three ingredients were measured, mixed and then saline was added. The whole was stirred for one minute on a Mini Max (Barnstead/Thermolyne, Iowa, USA). The formulation was always prepared the same day it was used. Alternatively, the first three ingredients can be mixed and stored at 4°C for several days (tested up to 14 days) with saline added just before use. The formulation is a milky white emulsion.
Growth Inhibition Assay
For the purpose of our assays, we aerosolized farnesol formulation as described in "Farnesol content measurement" below. However, this time the filter extraction was performed with ethanol. The ethanol extracts were assayed for farnesol contents using HPLC and then diluted (over the range between 120 and 1.87 mM). Mixed ethanol/medium (ETOH) and medium alone (media) were used as controls. ETOH concentration was consistently adjusted to match with that of the formulation in each experiment.
Non-small cell lung cancer cells (H460 and A549) were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI-1640 supplemented with 10% heat-inactivated FCS (Invitrogen Life Technologies, ON, Canada), 2mM L-glutamine, and 1% penicillin-streptomycin mixture, and grown at 37°C in a humidified incubator with 5%CO2. Cells in suspension of 20,000 cells/100mL of medium were plated to each well of a flat-bottomed 96-well plate, allowed to grow overnight and treated the next day with seven different concentrations of nebulized farnesol. Twenty-four hours later, 50mL of XTT labeling mixture (5ml labeling reagent with 0.1ml electron labeling reagent) was added into each well to a final XTT concentration of 0.3mg/ml and incubated for 4 hours in a humidified 37°C incubator. The colormetric XTT change quantifies cytotoxicity. The absorbance of the orange formazan dye product was directly quantified using a Benchmark® microplate reader (Bio-Rad Laboratories, CA, USA) read at 492nm with reference wavelength 650nm. The percentage of cell survival was calculated as follows: 1- (optical density of treated cells/optical density of untreated cell control) x 100. Each data point is the average result of three wells in two independent experiments.
Nebulization
A volume fill of 5 ml farnesol solution was nebulized with Pari LC Star and LC Plus (vented, valved jet nebulizers) driven by a Proneb Ultra compressor (Pari, Starnberg, Germany).
For determination of the output of farnesol, the nebulizers were connected directly to two Respigard filters (Marquest Medical Products, Englewood, CO) and a breath simulator with average flow rate of 18 L/min and tidal volume of 0.75 L. "Run time" of the nebulizers was defined as the time duration from the start of nebulizer to the time when there is no aerosol output for 15 seconds. The nebulization efficiency of farnesol formulation is defined as the total output of drug (farnesol) collected on the filters from the nebulizer calculated as a percentage of the total submitted to nebulization.
Particle size measurement
Particle size was measured using a Phase Doppler Anemometer (PDA) (Dantec Electronics Inc., Mahwah, NJ, U.S.A), as described by Prokop et al. (16) during tidal breathing. This procedure allows measurement of aerosol directly "inhaled" by the breathing machine from the nebulizer with time-varying flow rates and without spurious droplet evaporation. To determine the aerosol size distribution, the nebulizer was run continuously and 10 sec of data were collected for every minute of nebulization.
Farnesol content measurement
After the inhaled aerosol was collected on filters, the filter contents were extracted with 10 ml methanol. The methanol extracts were assayed using HPLC. Commercial farnesol is 96% pure and, fortunately, contains a natural tracer that has a UV absorbance at l=238 nm. We used this property of commercial farnesol in our HPLC assays.
The assays were performed on a Varian Prostar HPLC system with Varian Microsorb-MV100-5C8, 250 × 4.6 m column; detector-uv, 238nm, range 0.1; FR =1.2 ml/min, Methanol 100%; pressure 1088-1102 psi; retention time rt=3.78 min. The linear region corresponds to 310-1380 mg/ml farnesol. All samples were analyzed in duplicate.
Deposition and airway surface liquid model
To predict drug concentrations in the airway surface liquid, a numerical lung deposition model was employed as described in Lange et al. (17) (see also Finlay et al. (18)). In this model, the aerosol particles were followed as they traveled through the airways, in what is termed a one-dimensional Lagrangian approach, or compartmental method. The lung of a healthy adult was represented by a symmetrically branching model for the conducting airways (generations 0-14). Amounts of aerosol depositing in each generation of these lungs were estimated using the equation of Chan and Lippmann (19) for inertial impaction, Pich (20) and Heyder and Gebhart (21) for sedimentation and Gormley and Kennedy (22) for diffusion. Hygroscopicity can be included with the model but is negligible here due to the high aerosol mass fraction produced by the present nebulizers (Finlay (23)). A tidal volume of 0.75 liter and mouth breathing flow rate of 18 L/min were used. The volume of airway surface fluid in each generation of the conducting airways was estimated using the procedures of Lange et al. (17). This involves estimating the thickness of the mucous layer using mass conservation with models of average mucous velocity and production rate for each generation, based on fitting of in vivo clearance data and predicted deposition. Typical mucous concentrations for mucus production rates of 10 and 20 ml/day with tracheal mucous velocity 5 and 15 mm/in were chosen in our calculations, respectively, according to references (24).
Statistics
ANOVA was performed using SYSTAT (Evanston, Ill) with significance assumed at p < 0.05.
Results
Particle sizing experiment
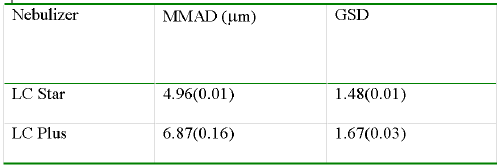
Particle sizes during tidal breathing from the tested nebulizers are shown in Table 1. The mass median aerodynamic diameter (MMAD) with the LC Star is approximately 5 mm, while the LC Plus produced somewhat larger droplet sizes.
Table 1: Particle Size During Tidal Breathing Given As Mean ± S.D. (N=3)
Inhaled farnesol
Percentages and amounts of inhaled farnesol from the nebulizers are shown in Table 2. All nebulizers produced approximately 30% total inhaled aerosol during the nebulization with no significant difference between nebulizers (p>0.05). The LC Plus has a significantly shorter run time (p<0.05).
Table 2: Amount Of "Inhaled" Farnesol Given As Mean ± S.D. (N=3)
Cytotoxic effects on lung cancer cells
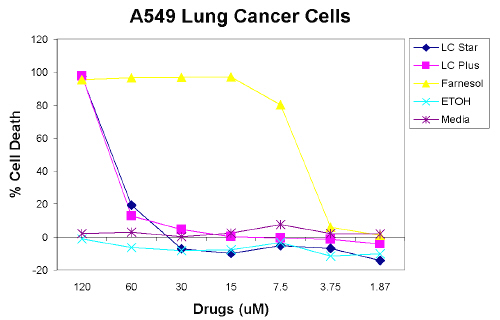
The cytotoxic effects of nebulized farnesol on two human lung cancer cell lines were compared by XTT assay. As seen in Figure 1, using A549 lung cancer cells, at concentrations above 120 mM the farnesol control, as well as farnesol nebulized by LC Star and LC Plus, all cause 100% cell death. The farnesol control has an IC50 of 4.5 mM. Farnesol nebulized by LC Star and LC Plus has an IC50 of approximately 70 mM, as shown in Figure 1.
Figure 1: Farnesol Causes Cell Death Of A549 Lung Cancer Cells. Cells Were Treated With Doses Of Nebulized Farnesol, Ethanol And Control Media For 24 Hrs. Cell Viability Was Quantified By XTT Assay. Each Data Point Represents The Mean Of Triplicate Wells.
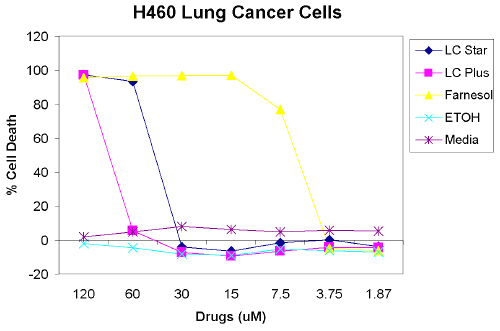
As seen in Figure 2, when testing H460 lung cancer cells, at concentrations above 120 mM, 100% cell death is also caused by the farnesol control and farnesol nebulized by LC Star and LC Plus nebulizers. Farnesol nebulized by the LC Star has an IC50 of 35 mM when using H460 lung cancer cells. Farnesol nebulized by the LC Plus is similar to the results using A549 lung cancer cell, as shown in Figure 2.
Figure 2: Farnesol Causes Cell Death Of H460 Lung Cancer Cells. Cells Were Treated With Various Doses Of Nebulized Farnesol, Ethanol And Control Media For 24 Hrs. Cell Viability Was Quantified By XTT Assay. Each Data Point Represents The Mean Of Triplicate Wells.
Simulation results from deposition and airway surface liquid model
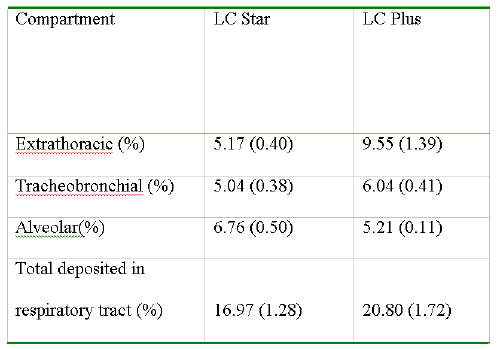
Using our measurements of the farnesol aerosol formulation, the deposited dosages of farnesol in an adult lung model for various nebulizers were estimated. A summary of the relative deposition grouped by region can be seen in Table 3.
Table 3: Relative Regional Deposition Of Farnesol For Adult Lung Deposition Model, Given As Mean ± S.D.
Estimated generational concentrations of farnesol in the airway surface liquid (ASL) immediately after completion of nebulization for various nebulizers, mucus production rates, and tracheal mucous velocities are shown in Figure 3. Generation 0 corresponds to the trachea. Results from these simulations are very similar; for example, the ratio of high/low concentrations is approximately 1.70 for both nebulizers as shown in Figure 3. Farnesol concentrations using the LC Plus nebulizers are apparently higher (1.2 times) than for the LC Star data. In general, farnesol concentrations at all locations are generally much higher than 120 mM or 27.28 mg/ml (molecular weight of farnesol is 227.37 g/mol) required for 100% cell death of lung cancer cells in vitro.
Figure 3: Estimated Generational Concentrations Of Farnesol In The Airway Surface Liquid Immediately After Completion Of Nebulization For Various Nebulizers, Mucus Production Rates, And Tracheal Mucous Velocities. Generation 0 Corresponds To The Trachea.
Discussion and conclusions
Our attempt to nebulize farnesol for the purpose of lung delivery is, to our knowledge, the first example of a sesquiterpene used in aerosol drug delivery research.
We have been able to demonstrate that it is possible to nebulize farnesol in a manner that retains its activity against cancer cell lines. Upon nebulization of our formulation in the LC Star and LC Plus, IC50 for both cell lines remained below approximately 70 mM. Nearly 100% of cells have undergone cell death at 120 mM farnesol concentration after nebulization in both the LC Star and LC Plus (A549 and H460). It should be noted that results regarding IC50 obtained on in vitro cancer cells are only an indication (not a guarantee) of success with chemosensitivity in vivo.
In summary, drug concentrations higher than IC50 in the airway surface liquid are predicted with our methods with LC Star and LC Plus nebulizers, suggesting in vivo trials of a formulation may be warranted with these particular nebulizers.
Acknowledgements
The authors are thankful to H. Orszanska (Aerosol Research Lab. of Alberta) for her laboratory help and C. Lange (Aerosol Research Lab. of Alberta) for his advice on the model calculation. The financial support of the Natural Sciences and Research Council of Canada is gratefully acknowledged.
REFERENCES
Sharma, S., White, D., Imondi, A.R., Placke M. E., Vail, D. M. and Kris M.G., Development of inhalation agents for oncology use. Journal of Clinical Oncology, 19: 1839-1847, 2001
Placke, M.E., Zimlich, W.C., Ding, J. Y., Westaway, D. J. and Imondi, A. R., Targeted aerosol therapy for the treatment of lung cancer. Respiratory Drug Delivery VIII, Tucson, Arizona, USA, 15-23, 2002.
Burke, Y.D., Stark, M. J., Roach, S.L., Sen, S.E. and Crowell, P. L., Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids, 32: 151-156, 1997.
Vigushin, D.M., Poon, G. K., Boddy, A., English, J., Halbert, G. W., Pagonis, C., Jarman, M. and Coombes, R.C., Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Chemother. Pharmacol, 42: 111-117,1998.
Ripple, G. H., Gould, M. N., Arzoomanian, R. Z., Alberti, D., Feierabend, C., Simon, K., Binger, K., Tutsch, K. D., Pomplun, M., Wahamaki, A., Marnocha, R., Wilding, G. and Bailey, H.H., Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clinical Cancer Research, 6: 390-396,2000.
Hudes, G. R., Szarka, C. E., Adams, A., Ranganathan, S., McCauley, R. A., Weiner, L. M., Langer, C. J., Litwin, S., Yeslow, G., Halberr, T., Qian, M. and Gallo, J. M., Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in Patients with refractory solid malignancies. Clinical Cancer Research, 6: 3021-3080, 2000.
Elson, C. E., Peffley, D.M., Hentosh, P. and Mo, H., Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer. Proceedings of The Society for Experimental Biology and Medicine, 221: 294-311, 1999.
Tatman, D., and Mo, H., Volatile isoprenoid constituents of fruits, vegetables, and herbs cumulatively suppress the proliferations of murine b16 melanoma and human HL-60 leukemia cells. Cancer Letters, 175: 129-139, 2002.
Rioja, A., Pizzey, A. P., Marson, C. M. and Thomas, N. S. B., Preferential induction of apoptosis of leukaemic cells by farnesol. FEBS Letters, 467: 291-295, 2000.
Anthony, M.L., Zhao, M. and Brindle, K. M., Inhibition of phosphatidylcholine Biosystheis following induction of apoptosis in HL-60 cells. The journal of Biological Chemistry, 274: 19686-19692, 1999.
Elson, C. E., Peffley, D. M., Hentosh, P. And Mo, H., Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer (Minireview). Proceedings of the Society for Experimental Biology and Medicine, 221:294-311, 1999.
Sharma, S., White, D., Imondi, A. R., Placke, M. E., Vail, D. M. and Kris, M. G., Development of inhalation agents for oncology use. J. Clinical Oncology, 19(6):1839-1847, 2001.
Miquel, K., Pradines, A. and Favre, G., Farnesol and geranylgeraniol induce acting cytoskeleton disorganization and apoptosis in A549 lung adenocarcinoma cells. Biochemical and Biophysical Research Communications, 225: 869-876, 1996.
Miquel, K., Pradines, A., Tercé, F., Selmi, S. and Favre, G., Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung ademocarcinoma A549 cells. The Journal of Biological Chemistry, 40: 26179-26186, 1998.
Prokop, R. M., Finlay, W. H. and Stapleton, K. W., An in vitro technique for calculating the regional dosages of drugs delivered by an ultrasonic nebulizer. J. Aerosol Sci., 26: 847-860, 1995.
Diot, P., Dequin, P. F., Rivoire, B., Gagnadoux, F., Faurisson, F., Diot, E., Boissinot, E., Le Pape, A., Palmer, L. and Lemarié, E., Aerosols and Anti-infectious Agents. Journal of aerosol medicine, 14: 55-64, 2001.
Lange, C. F., Hancock, R. E. W., Samuel, J. and Finlay, W. H., In vitro aerosol delivery and regional airway surface liquid concentration of a liposomal cationic peptide. J. Pharmaceutical Sci., 10:1647-1657, 2001.
Finlay, W. H., Lange, C. F., King, M. and Speert, D. P., Lung delivery of aerosolized dextran. Am J. Respir Crit Care Med., 161:121-127, 2000.
Chan, T. L. and Lippmann, M., Experimental measurements and empirical modeling of the regional deposition of inhaled particles in humans. Am Ind. Hyg. Assoc. J., 41:399-409, 1980
Pich, J., Theory of gravitational deposition of particles from laminar flows in channels. J. Aerosol Sci., 3:351-361, 1972.
Heyder, J. and Gebhart, J., Gravitational deposition of particles from laminar aerosol flow through inclined circular tubes. J. Aerosol Sci., 6:311-328, 1977.
Gormley P. G. and Kennedy, K., Diffusion from a steam flowing through a cylindrical tube. Proc. R. Irish Soc., 52A:163, 1949.
Finlay, W. H., Estimating the type of hygroscopic behavior exhibited by aqueous droplets. J. Aerosol Med., 11:221-229, 1998.
Wanner, A., Salathé, M., O’Riordan, T. G., Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med., 154:1868-190, 1996.
Corresponding Author: Warren H. Finlay, Aerosol Research Laboratory of Alberta, Department of Mechanical Engineering, University of Alberta, Edmonton, Alberta, Canada, T6G 2G8. warren.finlay@ualberta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps