J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(1):20-26, 2003
A preliminary investigation of chitosan film as dressing for punch biopsy wounds in rats.
Tanveer Ahmad Khan
Kulliyyah of Pharmacy, International Islamic University Malaysia, Kuantan. Malaysia.Kok Khiang Peh1
School of Pharmaceutical Sciences, University of Science Malaysia, Penang, Malaysia.Received 25 September 2002, Revised 12 December 2002, Accepted 7 February, 2003
PDF version
Abstract
PURPOSE. To investigate the wound healing efficacy of two chitosan films, Chit-AA and Chit-LA, in comparison with a commercial preparation, Omiderm®, using punch biopsy wounds in rats. METHODS. The punch biopsy wounds were created in the abdominal region of male Wistar rats. The films were evaluated in terms of transparency, flexibility, adherence property, ease of removal from wounds without damaging underlying tissues and fluid accumulation. In addition, the wounds were examined for dryness, exudation, contraction, period of epithelialization and scar formation. RESULTS. Chit-AA, Chit-LA and Omiderm® films were comparable in terms of transparency, flexibility, adherence property, ease of film removal from wounds without damaging underlying tissues and fluid accumulation. Although there was no statistically significant difference in wound dryness and exudation between the film treated wounds and untreated wounds (Control), a significant difference was obtained in complete wound closure (t100%), period of epithelialization and scar formation. CONCLUSIONS. Both Chit-LA and Chit-AA were able to promote wound healing with minimal scar formation.
Introduction
The merit of attempting to isolate a wound from the environment has long been appreciated, at least in the sense of providing physical and aesthetic protection. Almost all types of occlusive dressings act as a protective membrane and prevent desiccation, but they differ in their important physical properties. Occlusion accelerates epithelialization of surgically excised partial-thickness wounds (1). However, highly occlusive dressings tend to cause tissue maceration. During the healing process, the wound bed may be regarded as an open tissue-culture system threatened by bacterial spoilage and marked for death due to desiccation or asphyxiation for lack of blood vessels that provide oxygen and efficient gaseous exchange. A contact cover in the form of dressing or film is therefore required to keep invaders out and allow for moist wound healing, which expedites keratinocyte migration and natural wound closure.
The wound cover or dressing should preferably be inexpensive, readily available with minimal storage requirements and long shelf-life (2). It would also be an advantage for the material to be hemostatic, transparent (to indicate the presence of infection), and biodegradable as it restores normal function to the skin. Chitosan has been shown to have hemostatic activity and proposed for use as a topical agent in tissue repair (3). Chitosan in the form of powder has also been used as a wound bandage for subcutaneous wound healing in rats (4).
The development, characterization and biological evaluations of chitosan films have been described in earlier paper (5). The aim of the present study was to evaluate the wound healing efficacy of these films. Punch biopsy wounds were created on male Wistar rats. Clinical evaluations were performed to examine the film transparency, flexibility, ease of film removal from wounds without damaging underlying tissues, fluid accumulation, dryness of wounds, wound exudation, degree of wound contraction, period of epithelialization and scar formation. Omiderm®, a commercially available wound dressing preparation was selected for comparison.
MATERIALS AND METHODS
Materials
Chitosan (practical grade) was purchased from Sigma Chemical, St. Louis. USA. Glacial acetic acid was supplied by R & M Marketing, Essex, U.K. Lactic acid 85% A.R. and absolute alcohol were obtained from Ajax Chemicals, Auburn, Australia. Sagatal Injection (pentobarbitone sodium B.P. 60mg/ml) was purchased from Rhone Merieux, Essex, U.K. Omiderm® was purchased from ITG Laboratories, CA, USA. Sodium chloride solution (0.9% w/v) was a gift from B. Braun Pharmaceuticals, Penang, Malaysia. Propax (gauze swabs B.P) was supplied by Smith & Nephew Health Care, Selangor, Malaysia. Hypafix (dressing retention sheet # 4210) was purchased from Fisch-Smith & Nephew Laboratories, France. All other reagents and solvents used were of analytical reagent grade. The materials were used as received.
Preparation of Chitosan Films
The preparation method of Chit-AA and Chit-LA films was described in earlier paper (5).
Animals
Twelve male Wistar rats weighing between 250-300 g were used in the study. The rats were housed individually in a cage, with free access to water and commercial rodent chow throughout the study. The study was conducted in accordance with Guide to the Care and Use of Experimental Animal Care and approved by Institutional Animal Care and Use Committee of University Science of Malaysia.
Creation of Punch Biopsy Wounds
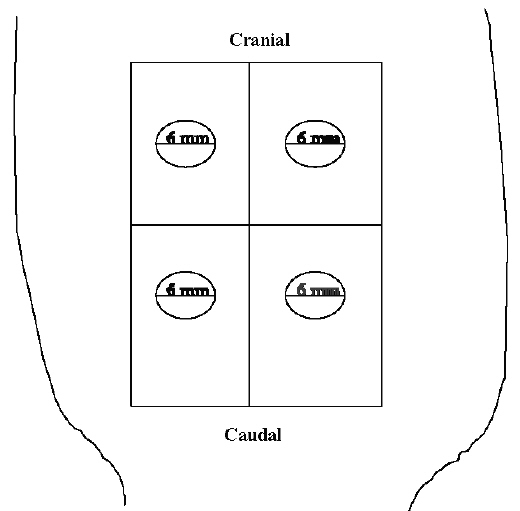
The rat was anesthetized with pentobarbitone sodium (50 mg/kg body weight) administered intraperitoneally. The animal abdomen was clipped with electric clippers followed by scrubbing the skin with 70% ethanol and normal saline. A full-thickness circular open wound was generated according to the method reported in the literature (1, 6, 7), in the abdominal region using a 6-mm sterilized punch biopsy (Stiefel, Germany) in a cranial-caudal direction (Figure 1). A total of four wounds were created on each rat. All freshly created wounds were washed with normal saline before the application of the films.
Figure 1: The Schematic Illustration of Locations of Punch Biopsy Wound (6 mm, diameter) generated on Abdomen of Rats.
Application of Films and Dressing Procedures
After wound creation, Chit-AA, Chit-LA, Omiderm® and Control, were randomly applied onto the four wounds of the same rat to eliminate inter-individual differences. The Control comprised gauze soaked with normal saline. As twelve rats were used in the study, there were twelve wounds for each treatment. The films and the Control were placed in such a way that the wounds could be completely covered. All the wounds were then covered with non-adherent occlusive gauzes to hold the films in place and further occluded with hypoallergenic adhesive tape. Finally, a bandage was wrapped around the trunk of the animals to protect the dressings. The bandage and the films were changed every three days until the wound had completely healed.
Examination of Punch Biopsy Wounds
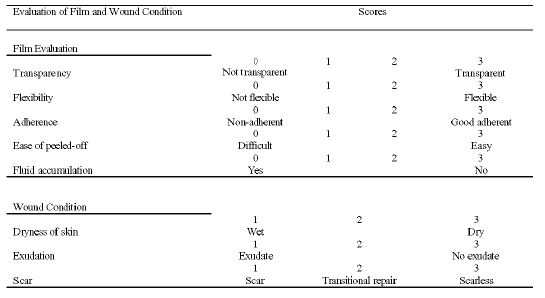
All the wounds and films were coded and not known to the evaluator. Conditions of the wounds were examined and photographs were taken at 0 (before film application), 3, 6, 9 and 12 days after wound creation until complete healing. Prior to wound evaluation, the bandage and the films were removed. The films were examined in terms of transparency, flexibility, adherence, ease of film removal from the wound without damaging underlying tissues and fluid accumulation, using the scores of 0-3. A score with a maximum of 3 was given to the desired attributes of films as wound dressing (transparent, flexible, good adherence, easily removed from wounds without damaging underlying tissues and no fluid accumulation), where 0 represented bad, 1 represented poor, 2 represented moderate and 3 represented good.
For the evaluation of wounds, the wounds were rinsed with 5 ml of normal saline from a syringe, gently wiped, and graded using the scores of 1-3 for dryness of wounds, exudation and scar formation. A score with a maximum of 3 was given to conditions that were preferable in wound healing (dry skin, absence of exudates and no scar formation), where 1 represented poor, 2 represented moderate and 3 represented good. The parameters investigated and their corresponding scores are given in Table 1.
Table 1: Protocols for the Evaluation of Punch Biopsy Wounds in Male Wistar Rats.
In addition, the outer margin of the wound was traced using a tracing paper and the area determined planimetrically. The degree of wound contraction (expressed as a percentage) was calculated using the following equation (1):
[(A Day 0 - A Day X ) / A Day 0 ] x 100 (1)
where X = 3, 6, 9, 12-day after wound creation, A = wound surface area.
The period of epithelialization of the wound was also estimated and expressed as the number of days taken for complete epithelialization with no raw wound left (8).
Wounds were graded as scar, transitional repair and scarless. Scar wounds showed the absence of hair follicles with thick, parallel collagen bundles that were apparently distinguishable from the surrounding dermis. Transitional repair wounds depicted an area with a decreased number of hair follicles or subtle dermal changes that did not fulfill scar criteria. Scarless wounds showed regeneration of hair follicles and a normal reticular collagen pattern that was indistinguishable from normal, unwounded tissue except for India ink (9).
Statistical Analysis
The results obtained from parameters, film transparency, flexibility, adherence, ease of film removal from the wound without damaging underlying tissues, fluid accumulation, dryness of skin, exudation, presence/absence of scar and period of epithelialization, were analyzed using a non-parametric Kruskal-Wallis test. On the other hand, the degree of wound contraction and time taken for complete wound closure (t100%) were analyzed using a one-way analysis of variance. When a statistically significance difference was obtained at p < 0.05, Tukey-HSD multiple comparison test was then performed.
RESULTS AND DISCUSSIONS
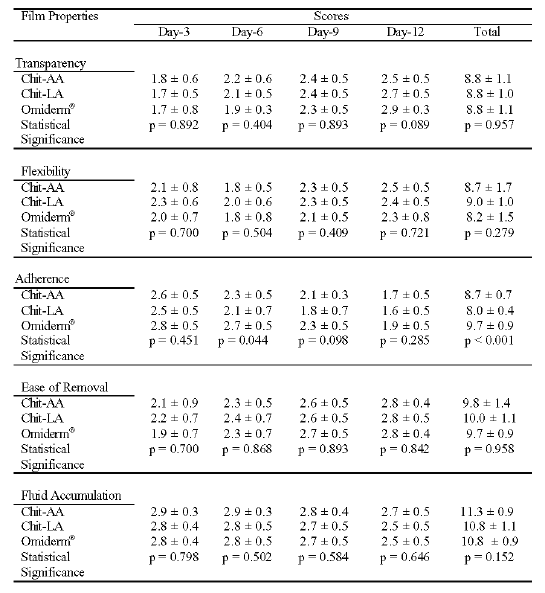
The transparency, flexibility, adherence, ease of film removal and fluid accumulation of Chit-AA, Chit-LA, and Omiderm® films are tabulated in Table 2.
Table 2: The Film Transparency, Flexibility, Adherence, Ease of Removal from Wounds and Fluid Accumulation for Chit-AA, Chit-LA and Omiderm®. Mean ± SD, N = 12.
The transparency property is one of the essential attributes of films for wound dressing as this allows wound to be monitored closely ensuring timely dressing change.The films became more transparent over time as the wound started to heal. No significant difference was observed in terms of transparency among the three films. The results of the flexibility of all the three films suggested that the films became more flexible over time as the wound started to heal. No significant difference was observed in the flexibility when the three films were analyzed statistically. The dressing must be rapidly and uniformly adherent as well as conform to wound bed topography and contour to prevent air or fluid pocket formation. Good adherence reduces pain, facilitates decontamination, prevents peripheral channeling into wound by bacteria, and promotes bonding to tissues. From Table 2, the adherence of the films decreased with time as the wound started to heal. There was no significant difference among the three films at day-3, 9 or 12 except at day-6 after wound creation. In contrast, when the total scores were used for comparison, a significant difference was obtained. The dressing should be durable, flexible, easily applied and removed without inducing trauma during dressing change over healed areas in the wound bed. The three films could be easily removed from the wounds without causing any injury to the underlying tissues. There was no significant difference when the three films were compared for this parameter. Fluid accumulation for the three films appeared to decrease slightly from day-3 to day-12 as the wound started to heal. No statistically significant difference was obtained in fluid accumulation among the three films.
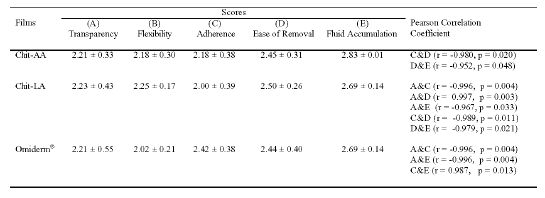
The relationship between parameters, transparency, flexibility, adherence, ease of film removal without damaging underlying tissues and fluid accumulation was analyzed using bivariate correlation statistical treatment and the results are given in Table 3.
Table 3: The Relationship between Transparency, Flexibility, Adherence, Ease of Removal and Fluid Accumulation for Chit-AA, Chit-LA and Omiderm®. Mean ± SD, N = 12.
For Chit-AA, a negative correlation was found between adherence and ease of film removal as well as ease of film removal and fluid accumulation. In the case of Chit-LA, negative correlations existed between transparency and adherence, transparency and fluid accumulation, adherence and ease of film removal, ease of film removal and fluid accumulation, but a positive correlation was observed between transparency and ease of film removal. A positive correlation existed between adherence and fluid accumulation, while a negative correlation was seen between transparency and adherence as well as between transparency and fluid accumulation for Omiderm®.
The results of wound dryness, exudation and contraction are given in Table 4.
Table 4: The Dryness, Exudation and Contraction of Wound for Chit-AA, Chit-LA, Omiderm® and Control. Mean ± SD, N = 12.
The dryness of film treated and untreated (control) wounds gradually increased with respect to time. The wound improved from wet to finally dry when healing was near to completion. There was no statistically significant difference between the treated and untreated wounds in the dryness of wound when they were compared at day-3, 6 or 12 after wound creation, but at day-9, a statistically significant difference was obtained. When the total scores were used for comparison, the four treatment groups did not exhibit statistically significant difference in wound dryness although the film treated wounds dried at a faster rate than those of the Control. The presence of exudates on the wounds is critical as it could delay wound healing. There was no statistically significant difference in exudation of wounds among the treatment groups, indicating that the exudation of the film treated wounds did not differ from those untreated wounds (Control).
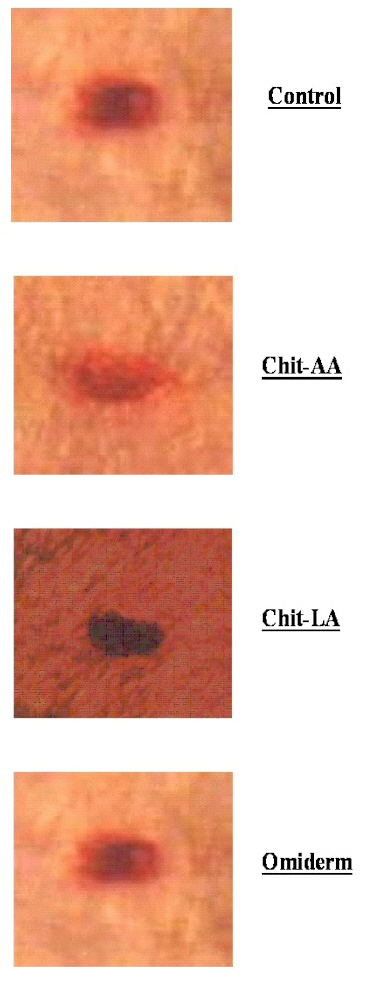
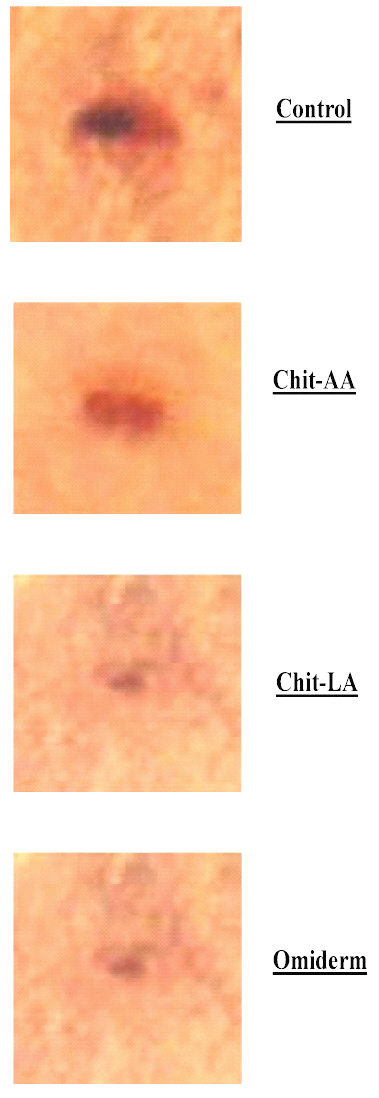
The wound contraction after applications of Chit-AA, Chit-LA, Omiderm® and Control are shown in Figures 2a-e, while the mean wound contraction results for the four different treatment groups are tabulated in Table 4.
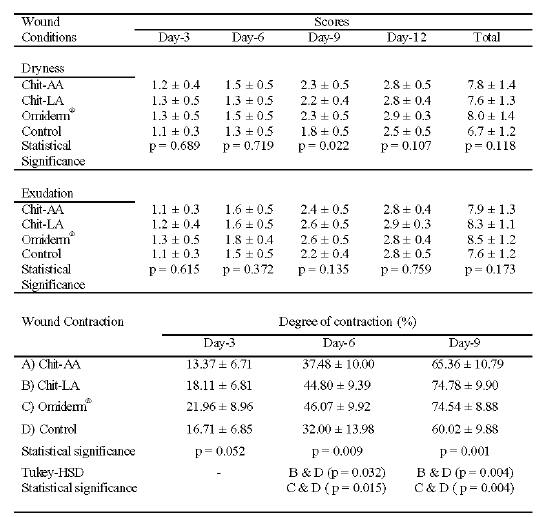
Figure 2a: The Photographs Showing the Typical Changes of Wound Contraction at day-0 After Wound Creation for Chit-AA, Chit-LA, Omiderm®, and Control.
Figure 2b: The Photographs Showing the Typical Changes of Wound Contraction at day-3 After Wound Creation for Chit-AA, Chit-LA, Omiderm®, and Control.
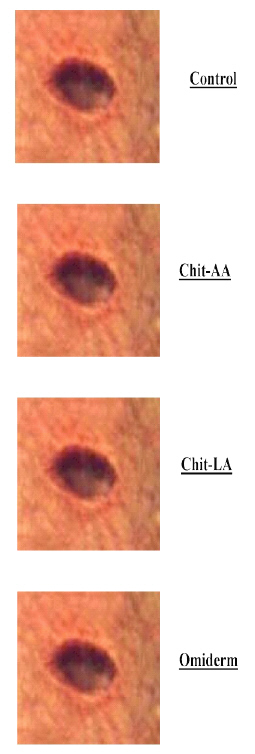
Figure 2c: The Photographs Showing the Typical Changes of Wound Contraction at day-6 After Wound Creation for Chit-AA, Chit-LA, Omiderm®, and Control.
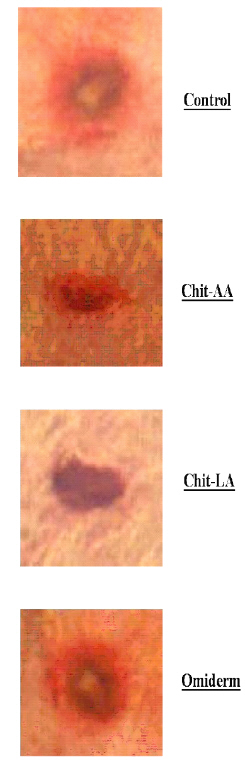
Figure 2d: The Photographs Showing the Typical Changes of Wound Contraction at day-9 After Wound Creation for Chit-AA, Chit-LA, Omiderm®, and Control.
Figure 2e: The Photographs Showing the Typical Changes of Wound Contraction at day-12 After Wound Creation for Chit-AA, Chit-LA, Omiderm®, and Control.
The wounds contracted gradually over the treatment period. When the wound contraction of the four different treatment groups were compared, no significant difference was observed at day-3. A significant difference was only obtained at day-6 and day-9. Nevertheless, the contraction of wounds treated with Chit-AA did not differ significantly from the untreated wounds up to day-9.
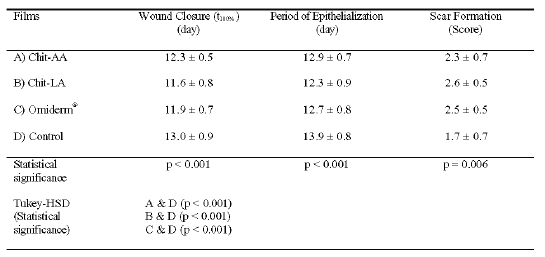
The average time taken for complete closure of the wounds (t100%) is shown in Table 5.
Table 5: The Time Taken for Complete Closure of Wound, Period of Epithelialization and Scar Formation for Chit-AA, Chit-LA, Omiderm® and Control. Mean ± SD, N = 12.
Almost all the wounds healed completely between 11-14 days. There was a significant difference in the t100% values among the treatment groups. The film treated wounds healed in a significantly shorter duration than that of the Control, indicating that Chit-AA, Chit-LA and Omiderm® were effective and beneficial for wound healing. Although the treated wounds healed first with Chit-LA followed by Omiderm and lastly Chit-AA, post-hoc test showed that the three films were comparable in wound healing efficacy. These findings suggest that Chit-AA required a longer period to demonstrate wound healing effect compared to Chit-LA and Omiderm.
The period of epithelialization for the treated and untreated wounds (Control) as shown in Table 5 differed significantly. A decrease in the period of epithelialization of treated wounds compared to those of Control could be attributed to an increase in the rate of wound healing. A positive correlation coefficient of 0.974 (p = 0.026) was obtained between the period of epithelialization and wound contraction, indicating that epithelialization promotes wound contraction enabling the wound to heal faster. Excised wounds generally healed by a combined process of contraction and epithelial migration. During the proliferative phase of the wound healing process, wound contraction occurred due to the presence of specialized contractile cells, myofibroblasts, which pulled the wound edges together leading to closure of the defects (10).
Wound scar tissue was rigid and formed by fibroblast proliferation and formation of dermal collagen and ground substance, and thus might be thick and firm in texture (11). The scar formation data of wounds treated with Chit-AA, Chit-LA, Omiderm® and Control are depicted in Table 5. There was a statistically significant difference in the scar formation between film treated wounds and Control. It was observed that no scar was seen in 41.7% of wounds (5 over 12 wounds) for Chit-AA, 58.3% (7 over 12 wounds) for Chit-LA, 50.0% (6 over 12 wounds) for Omiderm® and 8.3% (1 over 12 wounds) for the Control.
The cosmetic appearance of scars can often cause psychological distress. Scar tissue showed an aberrant architecture, with large parallel-organized collagen bundles and a scattered elastin fibre network, which explained its rigid characteristics (6). In general, as the wound size increased, the scarless repair ability decreased as shown in the fetal wounds (9).
Increasing wound size was strongly associated with a decrease in the frequency of scarless repair and an increase in the frequency of scarring. This was because larger wounds produced a greater surface area that was filled with fibrin, fibronectin, and other matrix proteins. Keratinocytes and fibroblasts bind to these provisional, extracellular matrix proteins via cell surface integrin receptors. These cell-matrix interactions regulate motility, proliferation, and cell signaling events. Theoretically, larger wounds may prolong these cell-matrix adhesion events, leading to greater platelet activation and degranulation, and a more exuberant release-synthesis of potentially scar-inducing growth factors for instance transforming growth factor-b or platelet-derived growth factor. On the other hand, smaller excisional wounds, which re-epithelialize more rapidly, may produce a more limited cytokine response that permits scarless repair to occur.
The results suggested that chitosan film treatment might have beneficial influence on the various phases of wound healing such as fibroplasia, collagen synthesis and contraction resulting in faster healing. It was possible that the enhanced healing of wounds in rats by chitosan film was a result of its stimulating activity and/or its capacity to stimulate fibroblast proliferation resulting in the progression of wound healing (3). It was also suggested that chitosan might induce fibroblasts to release interleukin-8, which is involved in migration and proliferation of fibroblasts and vascular endothelial cells (12).
CONCLUSIONS
Chit-AA, Chit-LA, and Omiderm® films were comparable in parameters, transparency, flexibility, adherence, ease of film removal from wounds without damaging underlying tissues and fluid accumulation. The four treatment groups exhibited no statistically significant difference in wound dryness and exudation. However, the film treated wounds were significantly different from the untreated wounds (Control) in complete wound closure (t100%), period of epithelialization and scar formation. Both Chit-LA and Chit-AA films were able to promote wound healing. Nevertheless, the skin irritation effect of Chit-AA as reported in our earlier findings (5) may compromise its use as wound dressing.
References
Agren, M.S., Mertz, P.M. and Franzen, L., A comparative study of three occlusive dressings in the treatment of full-thickness wounds in pigs. J Am Acad Dermatol, 36:53-58, 1997.
Widra, A., Skin, Synthetic, in Mark, H.F., Bikales, N.M., Overberger, C.G. and Menges, G. (eds), Encyclopedia of Polymer Science and Engineering. 2nd ed., vol.15, John Wiley and Sons, USA, pp 335-344, 1989.
Diegelmann, R. F., Dunn, J. D., Lindblad, W. J. and Cohen, I. K., Analysis of the effects of chitosan on inflammation, angiogenesis, fibroplasia, and collagen deposition in polyvinyl alcohol sponge implants in rat wounds. Wound Rep Reg, 4:48-52, 1996.
Sathirakul K., How. N.C., Stevens. W. F. and Chandrkrachang. S., Application of chitin and chitosan bandages for wound healing. Adv Chitin Science, 1:490-492, 1996.
Khan, Tanveer. A., Peh, K.K. and Ch’ng, H.S., Mechanical, bioadhesive strength and biological evaluations of Chitosan films for wound dressing. J Pharm Pharmaceut Sci, 3(3):303-311, 2000.
De Vries, H.J.C., Zeegelaar, J.E., Middelkoop, E., Gijsbers, G., Van Marle, J., Wildevuur, C.H.R. and Westerhof, W, Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes. A clinical study. Br J Dermatol, 132:690-697, 1995.
Mattioli-Belmonte, M., Nicoli-Aldini, N., De Benedittis, A., Sgarbi, G., Amati, S., Fini, M., Biagini, G., Muzzarelli, R.A.A., Morphological study of bone regeneration in the presence of 6-oxychitin. Carbohydr Polym, 40:23-27, 1999.
Chithra, P., Sajithlal, G.B. and Chandrakasan, G., Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol, 59:195-201, 1998.
Cass, D.L., Bullard, K.M., Sylvester, K.G., Yang, E.Y., Longaker, M.T., and Adzick, N.S., Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg, 32(3):411-415, 1997.
Miller, T. A., The Healing of Partial-Thickness Skin Injuries, in Hunt, T. K., (ed), Wound Healing and Wound Infection: Theory and Surgical Practice Appleton-Century-Crofts, New York, pp 81-98, 1980.
Lawrence, C.M. and Cox, N.H., Physical Signs in Dermatology, Color Atlas and Text. Mosby-Year Book Europe Limited, London, 1993.
Mori, T., Okumura, M., Matsura, M., Ueno, K., Tokura, S., Okamoto, Y., Minami, S., Fujinaga, T., Effect of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials, 18:947-951, 1997.
Corresponding Author: Kok Khiang Peh, School of Pharmaceutical Sciences, University of Science Malaysia, 11800, Penang, Malaysia. kkpeh@usm.my
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps