J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 6(1):84-94, 2003
Cost-savings from subsidized pro-active pharmacist interventions.
Stephen Law1
Department of Economics, Mount Allison University, Sackville, New Brunswick, CanadaWeiqiu Yu
Department of Economics, University of New Brunswick, Fredericton, New Brunswick, CanadaReceived 10 October 2003, Revised 24 March 2003, Accepted 09 April 2003
PDF version
Abstract
PURPOSE. This paper evaluates a pilot project to determine the desirability of implementing a reimbursement model for pro-active interventions. A drug plan administration conducted an experiment in which a pharmacist could recommend to physicians the substitution of lower-cost therapies with equivalent health outcomes. The pharmacist shared any cost savings with the insurer. METHODS. Drug plan costs without the intervention were estimated using time-series forecasting models and compared to actual costs with the intervention. RESULTS. Over the course of this experiment, there were some cost savings generated by reactive pharmacist interventions but pro-active interventions, intended to influence subsequent physician behaviour, appear to have had no significant effect on the profile of drug expenditures. CONCLUSIONS. The evidence does not lend extensive support for full implementation of this type of reimbursement model.
Introduction
This paper presents results from a pilot project conducted by a third-party insurer to investigate a strategy for introducing greater price-responsiveness to the consumption of prescription pharmaceutical products. The pilot project involves a target pharmacy and a clinic of physicians, located at the same site in New Brunswick, Canada. The pharmacist provides information concerning prescription alternatives to the physicians about three selected classes of drugs. The pharmacy could initiate an intervention in a reactive or in a pro-active fashion. A reactive intervention is undertaken after a prescription has been written. Upon receiving the prescription, the pharmacist recognizes the potential for improved therapy and initiates one of the following: a non-fill of the prescription, a change of medication, or a termination of therapy. Resulting cost savings are shared equally by the pharmacy and the third-party insurer. In contrast, a pro-active intervention involves a scheduled visit by the pharmacist to the physician, and possibly subsequent reinforcing contacts, to present and review therapeutic alternatives to influence prescription behaviour at the point of prescribing.
The purpose of the pilot is to develop an alternative reimbursement process to encourage pharmacists to collect and disseminate information that may result in cost savings while not reducing patients' health outcomes. This experiment is based on the premise that physicians and pharmacists have different information sets regarding pharmaceutical products and is intended to investigate the impact, on the drug expenditures of the third-party insurers who would likely be the primary supporters of such a process, of the provision of additional information to physicians by pharmacists.
We use data collected from the pilot project to estimate cost savings to the third-party insurer. The report from the original project has not been publicly released. Cost savings from pro-active interventions are assessed on a counter-factual basis, that is, the cost of drug utilization in the absence of the interventions is estimated and compared to the actual cost with the interventions under the pilot project. In addition, we conduct regression analysis to account for the impacts of changes in other relevant variables on total cost.
The results indicate that over the course of this experiment, there were some cost savings generated by reactive interventions but pro-active interventions appear to have had no significant effect on the profile of drug expenditures.
Drug costs and the market for pharmaceutical drugs
The simplest expression of drug expenditures is the decomposition of those costs into price and quantity components. Since most policies that might act to constrain pricing are set at the national level in Canada, aside from modifications to the structure of dispensing fees there is typically little scope for state or provincial health policy authorities to impact costs through price adjustments for a particular drug.[1] However, some cost reductions can be achieved on the quantity side through substitution of drugs: the pilot project is based on the supposition that this principle can be extended beyond the substitution of bio-equivalent products to the substitution of therapeutically-equivalent products. The substitution of lower-priced bio-equivalent products (usually "generic" substitution) is required of pharmacists (without the necessity of a new prescription) under the policies of the third-party insurer in the region where this experiment was undertaken. However, therapeutic interchange - when a drug that is not biologically equivalent is substituted for the originally prescribed drug - requires the authorization of a physician or, essentially, a new prescription. The basis for the cost savings is a reduction in the use of higher-priced products or therapies through therapeutic interchange. Since it is imperative that any purposive adjustment must not involve adverse consequences for health outcomes, generally the case implies increasing quantities of less costly products used as substitutes. Hence, the focus is on therapeutic-equivalence. If this equivalence is best determined at the level of the client, this type of intervention requires consultation between pharmacist and physician to ensure the quality of care. One motivation for the pilot project is to investigate a method for controlling prescribed quantities of target drugs, with a focus on physician education.
Typically, in the prescription drug market neither patients nor physicians directly bear the full cost of the utilization decision, resulting in a failure of price to act as a signal to encourage more restrained use of drug resources. The major purchasers of prescription drug products are public and private health care providers or insurers who can exert only a limited influence on consumption choices.
The pilot project was proposed to investigate one method for enhancing the price-responsiveness of the demand for pharmaceutical products by providing an incentive for pharmacists to communicate price and treatment information to the physicians who choose the products for which a third-party insurer will pay, and which its clients will consume. The pilot project was initially proposed, in part, by the operator of the target pharmacy.
Information in the prescription process
Following medical training, the publications of medical research schools and the publications of the medical association to which a physician belongs are, for most prescribers, the authoritative sources of new information on prescribing practice. However, "formal medical education provides too few courses in practical therapeutic decision-making and does not emphasize the comparative cost-effectiveness of different drugs for specific clinical problems, or the necessity for the critical analysis of the drug-evaluation literature that is utilized in promotional efforts." [2] This lack of focus on cost-effectiveness is mirrored in primary information sources for many physicians that are concerned with effects and dosages, such as the Compendium of Pharmaceuticals and Specialties (CPS), [3] that were not initially designed to disseminate information on the relative costs of different drugs. [4]
Any changes to prescribing practice that a practising physician may undertake will be because of information received or developed by the physician after formal training. However, the process of acquisition of information, assessment of the gathered information, evaluation of prescribing behaviour, and then implementation of any resulting behavioural changes is time-consuming and costly.[5] One result of information costs, verified by empirical research, is that a significant determinant of prescribing behaviour is the length of time since a physician's formal training. If information is expensive, especially with respect to the time required for gathering and processing, and the resources and incentives of physicians are insufficient to support a high level of effort devoted to upgrade and implementation then there is the risk that the information sets of physicians may be less complete than is desirable. Physicians, quite properly, are primarily concerned with the "dosages-and-adverse-effects" type of information that is likely to be found in, e.g., the CPS. However, the supplementary concern of cost-effectiveness appears to be under-addressed. In particular, physicians may not be well informed about the prices of the therapies they prescribe.
The Kolassa study reporting results of a survey conducted in 1995 found that physicians were generally not able to estimate with sufficient accuracy the prices of commonly prescribed drugs and concluded that "the inadequacy of the [physicians' drug] cost estimates must lead to questions of the adequacy of any cost-containment measures that do not include the attainment of accurate price knowledge by physicians and physician commitment to consider this factor in making decisions." [6] A notable feature of this survey of physicians' perceptions of prescription drug prices are the physicians responses to questions about information provided by pharmacists: on a scale where 1 = "Strongly Disagree" and 7 = "Strongly Agree", the mean response to "Pharmacists are a good source of price information" was 5.1 but the mean response to "Pharmacists often contact me to recommend lower priced drugs" was 2.1. [6]
Although the Kolassa study surveyed physicians operating in a region (on another continent) with a distinct drug reimbursement program from the target area, it was a similar observation about the current prevalence, and potential benefits from increasing future prevalence, of physician - pharmacist contact over drug prices in the target area that prompted the pilot project. The Kolassa study concludes, "Attempts to control health care costs that do not focus on physician education in the area of treatment costs may prove ineffective." [6] However, there is "some evidence that general practitioner (GP) prescribing is affected by their perceptions of drug costs, at least within certain therapeutic groups" [7,] suggesting that while physicians may not be well informed about absolute prices, they may have some awareness of relative prices.
Concerning the information problem, the following factors are important in proposing solutions: the initial information set of physicians; the cost (particularly in terms of time) of upgrading that information; and the incentives for physicians to absorb the information and to implement any behavioural changes suggested by new information. Thus, it may not be sufficient simply to provide necessary information to physicians. Studies have shown that "dissemination of printed-educational materials alone - which represents rational-information strategy - rarely changed behaviour". [2] The additional components of the problem must be addressed if interventions are to deliver enough benefit to justify their cost. [8] Simple distribution of information may not secure the desired results. [2]
Private drug companies have long been aware that influencing prescribing behaviour requires more than the distribution of, for example, informative advertising leaflets. Private firms, motivated by cost concerns, would not expend resources for diffuse, face-to-face, dissemination of information unless such a practice delivered measurable benefits in the form of increased sales. Although the provision of information, or "detailing", which is undertaken by private firms is not without value, it may pose a problem if not matched with information from other sources. [2] There is considerable concern over the promotional activities of drug producers, especially at the administrative level, since the incentive structure for pharmaceutical firms dictates that successful detailing should lead to higher expenditures on drugs (higher sales revenue). In the absence of counter-vailing detailing from other sources, the impact of private detailing is mitigated primarily by the `learning-by-prescribing' experience of physicians. [9] And, further, "physician awareness of the profit motive and its potential impact on the objectivity of promotional messages presents a formidable obstacle for commercial representatives." [10] If neither of these mitigating factors counteracts the effects of private detailing to any great extent, then other interventions, such as counter-vailing detailing, will be considered by third party insurers. We turn now to the features of the distribution of third party information.
The level and type of intervention are critical determinants of the cost and effectiveness of interventions into the transmission of information regarding prescribing practices. Centralized interventions such as the distribution of information circulars, drug utilization review (DUR) letters, or small-area variation (SAV) results generally can be conducted at lower cost than diffuse interventions that typically require more intensive contact and greater commitment of resources. "For some drug-use problems (for example, correct antibiotic dosing), regulatory or administrative actions may be less costly than are educational strategies in reducing inappropriate drug use." [2] Both distribution (output) and information-gathering (input) are more expensive for a more diffuse approach. Yet some of the behavioural changes that result from detailing and from some DUR results appear to be best elicited through diffuse transmission networks since, as in some studies, "the increased costs of two face-to-face visits by clinical pharmacists in comparison with a print-only approach were more than outweighed by the increased saving over the less-intensive approach." [2, 11]
More complex forms of intervention are typically more expensive. The problem is to choose the right mix of centralized information dissemination and administrative rules versus diffuse information distribution and more individual discretion. The choice of intervention will depend on the cost of each strategy and the benefits desired since the more complex is the intervention environment, the less a centralized top-down approach is likely to deliver desirable behavioural changes, that is, a simple rule is not likely to generate valuable results when decisions have many dimensions. After a physician arrives at a diagnosis, the more complex is the prescription decision in response to that diagnosis, the more it is necessary to delegate prescription decision-making to the point of greatest information about the patient.
General guidelines for successful intervention choice may reflect the following considerations. (1) Where the decision to be made by the prescriber is uncomplicated an administrative approach may be most cost-effective - for example, for diagnosis X, prescribe drug A and not drug B. Information costs are minimized by centralized distribution and compliance may be enforced by rules, if necessary. Centralized or administrative responses are less expensive and likely to be suitable for prescription behaviour changes which have high incentive-compatibility and low information requirements. (2) Where the decision of the prescriber is relatively more complicated a diffuse approach may be most cost-effective - for example, for diagnosis X, prescribe drug A, unless the patient has a history of otherwise benign condition Y, in which case prescribe drug B, or unless the patient's case of X is very severe, in which case prescribe both drug A and drug C, unless the patient has a significant probability of being of type Z, in which case prescribe drug D, etc. The higher costs of face-to-face information diffusion may balance by higher benefits: in some complex cases, there may be no benefit at all to pursuing an administrative solution or it may be impractical or even dangerous, to address the situation by imposing one.
The pilot project relies on a diffuse mechanism of intervention. However, it is not known whether the target practices or drug classes chosen for the study are those that are most suited to this approach.
Drug utilization review (DUR) and small-area variation studies (SAV)
Drug utilization reviews can identify prescription practices that diverge from average practice. The implication is that divergent and costly practices should receive attention and perhaps be modified. However, unless there is corresponding analysis of underlying conditions, which may determine prescription profiles, a divergent practice may be assumed to be of the type that does not warrant a behavioural change since a physician with a divergent profile may also be treating a patient population that differs significantly from the average. This possibility will reduce the incentive to undertake changes where they are warranted.
Where DUR indicates the need for response, the mechanism of the distribution of DUR results impacts the potential benefits that can be achieved. DUR letters are more effective if distributed to both physicians and pharmacists, and reinforced by repeated interactions. [12] Further, use of DUR to elicit behavioural change is best accomplished within a framework that reduces the variability of the credibility of the information source, that is, the involvement of other physicians is often central: "If the general practitioners get insight into their drug prescribing presented by a medical colleague in a non-threatening fashion, they will improve cost efficiency and quality of their prescribing." [13] The more effective intervention is thus the more expensive option, requiring additional resources to secure the time of physicians to act as peer reviewers. Detailing is often presumed to be more effective than the distribution of DUR letters but the primary issue with both forms of intervention appears to be the type of contact rather than the format or content. Centralized distribution of DUR letters or third party information may serve when desired behavioural changes are uncomplicated and there are strong incentives for the change; detailing and/or peer review with DUR may be the intervention of choice when prescription decisions are more complex and incentives are more uncertain. An important function served by DUR is to inform insurers, who ultimately bear the cost of prescriptions, of the areas upon which educational efforts should focus, whether these efforts are through peer review or other reinforced personal contacts and whether or not the DUR results form part of the educational materials.
Like DUR, a small-area variation (SAV) study seems to be well suited for informing policy-makers or insurers of the most productive points for intervention... [5] The "variation detected by small-area variation analysis...requires explanations other than randomness, such as medical uncertainty, which can result from a lack or inadequate diffusion of information." [5] This observation appears to suggest that, the dissemination of SAV results is most productively accomplished through print media, as is current practice. Specific information, targeted to problems identified through SAV analysis, can then be further disseminated through more diffuse channels where this approach is warranted.
Detailing
The simplest definition of this activity is "providing details". There are a number of, partially conflicting, strategies for detailing efforts undertaken as part of a cost containment strategy. The program could target the worst offenders, in which case the educational efforts or the attention of informed parties such as peer reviewers would be targeted toward the worst offenders, the least-well-informed, or the most divergent practices (as identified by SAV or DUR). Alternately, the target could be big spenders. The membership of this group may include members of the first target group (worst offenders) but also may include those with prescription profiles with smaller divergence but larger volume or total expenditure. This approach mirrors a strategy observed in private detailing. [10] Another possibility for a detailing program could be to target opinion-leaders within the physician population. The membership of this group usually does not overlap with the membership of the first group. This strategy attempts to capture spill over effects to reduce expenditure on information provision and should be pursued when focussing educational efforts upon opinion leaders, likely to be the best-informed practitioners in a locality, will produce the most significant behavioural changes at least cost. [10] Cost-effective detailing requires some knowledge of the characteristics of prescribers. If it is the case that detailers are sufficiently well informed about the physician network in a target area, they should choose to direct detailing efforts to the most effective set of physicians.
However, sorting physicians into target groups may be an expensive or time-consuming process in itself. Of the following four possible target groups of physicians, sets 1 and 2, would be chosen with a view to maximizing benefits; sets 3 and 4 would be chosen to minimize costs: (1) the least-informed - for greatest potential individual gains; (2) the biggest spenders - for greatest potential volume reductions; (3) the best-informed or opinion leaders - for greatest spill over effects in education; (4) all physicians - for lowest (no) sorting cost. The net benefits of detailing for drug cost remediation can be significant. A 1986 study of university-based drug detailing realized cost-savings from this academic detailing of up to 13%. [11] The authors suggested that the ratio of benefits to costs, 1.8 in their study, could have been increased to at least 3.0 by choosing the higher-volume prescribers in set 2. Such savings are not always realized in practice, necessarily. One suggested cause of this failure is the use of non-academic (commercial) sources of information, implying that detailing efforts must confront the issue of the credibility of the details. The information source would not be the cause if all of the physicians in the target area (as distinct from the target group, if set 3 is chosen) were already well-informed physicians. This situation may imply that the issue for drug cost remediation is not information but incentives. Detailing is unlikely to result in significant expenditure remediation if there is little scope for behavioural changes, that is, if there are few substitutes for the expensive treatments covered in the distributed information. To pursue a detailing strategy there should be evidence a priori of potential savings. Less effective detailing methods, although less costly, also give rise to smaller benefits, if detailing was pursued through a mail-out rather than face-to-face contact with reinforcement, for example. Finally, poor detailing will deliver poor results, especially in cases where there is a failure of detailers to follow program protocol.
However, the overall savings in the health care system may be higher than direct drug expenditure reductions and thus it may be worth pursuing detailing campaigns that seem less cost-effective from the standpoint of drug cost containment alone. For example, additional indirect benefits may result as prescription interventions alert physicians and pharmacists to potential drug conflicts. Thus, although detailing or similar interventions may reduce costs, mitigating factors may reduce direct cost savings to a very low percentage and indirect cost savings may be difficult to assess.
Pharmacist information
One locus for detailing is the site of dispensing. The advantages of choosing this point of intervention are that the pharmacist may be well-informed about the local physician network, and could choose the most cost-effective set of physicians upon which to focus educational efforts; the pharmacist may be well-informed about the client base of the physicians and could identify those drug categories which may yield the greatest cost savings from detailing; and the pharmacist may engage in repeated interactions with physicians and could reinforce detailing messages. There are some drawbacks to pharmacist interventions, in addition to the expense associated with diffuse detailing efforts that exceeds that for more centralized information dissemination, which may limit their cost-effectiveness. "Disadvantages of this method include the fact that it is retrospective, labour-intensive, lengthy, seen by practitioners as `policing' drug use rather than optimising pharmaceutical care, and more reactive than pro-active." [14] However, consider the example of a study of a pharmacy-based antimicrobial-monitoring service, where the criteria for intervention were based on cost containment and concerns with inappropriate or excessive prescribing and cost, both physician and pharmacist rates of compliance exceeded 80%. The rate of pharmacist compliance averaged 90%, with a low of 79%, and regarding physician compliance with the prescribing directives: 85.9% met criteria; 9.2% did not meet criteria, but were approved; 2.4% were for drugs replaced by alternate therapy; 0.9% were dispensed without review (time delay); and 1.6% were overrides or inappropriate dispensing. [15]
The pilot project was initiated to investigate whether significant cost savings inevitably arise from interventions conducted at the point of dispensing.
Method
We assume that pharmacy characteristics or activities do not determine the number of clients (or patients) in the target area. That is, the number of people in the region of the pilot project, which includes both target and non-target pharmacies, who require a therapy involving prescription medicines is not a number that is influenced by the actions of the target pharmacy. The number of clients, we assume, is determined by eligibility for coverage, which is controlled by the third-party insurers in response to income indicators, and by disease incidence within the eligible population.
The potential beneficial result of an intervention is the substitution of a lower-priced therapy; not the substitution of a lower-priced version of the drug originally prescribed - since cost-reducing bio-equivalent substitutions are already permitted, or required, by law and regulation - nor a termination, nor exclusion. Terminations may occur as a result of reactive but not of pro-active interventions. We assume that if an eligible patient has a condition requiring treatment with a prescription drug then that person will submit some claim to the insurer. While the substitution of a therapy that does not require drugs may be possible in some cases, providing information about such substitutions is not in the financial interest of the pharmacy, nor is it the specific mandate of the pharmacy to provide this type of information under the pilot project. For "termination" to be applied to pro-active interventions, the result would be "exclusion", that is, the client who would have received treatment involving a prescription drug receives an alternate, non-drug, form of therapy following the intervention. Patients would thus be excluded from the client group. We have no evidence that there was significant exclusion. We assume that there is no significant substitution to non-drug therapies.
While we recognize that physicians may limit the pharmacists' ability to recommend an alternative drug or therapy by writing "no substitution" on the prescription note, we assume that this limitation is negligible since physicians were expected to cooperate fully with the pharmacists in this pilot project. The determination of which drugs qualified for therapeutic interchange was made by the target pharmacist, the third-party insurer, and physician and pharmacist consultants. Any materials for pro-active interventions were designed by the target pharmacist in consultation with the third-party insurer. We assume that the project participants selected the intervention drugs and materials as best they could, given informational resources typical to pharmacists operating in Canada. We seek to investigate the results of selection and intervention.
The target pharmacist has some incentive to devote effort to the intervention process. We do not assume that the returns to proactive interventions are necessarily larger than returns to other activities, only that gross returns are non-negative. A binding time constraint could result in little or no effort being devoted to intervention activities. The return to intervening can be derived from the expression for the share of the cost-savings that accrue to the pharmacist as a result of intervention effort. Under the pharmacist reimbursement system in place during the project, the insurer's expenditure per client, C, is:
(1)
where d = dispensing fee, m = pharmacy mark-up, p i = AAC = actual acquisition cost per unit for drug i ,
q i = quantity of drug i, and k = co-pay amount. In the discussion that follows, we set k = 0 since it is simply a transfer from the consumer to the insurer. Compare an initial treatment with acquisition cost p1q1 to one with a lower cost, p2q2. For one patient who is transferred from one treatment to another, the pharmacist receives a payment for the new drug plus half of the savings from the transfer as an incentive payment less any costs of transferring, that is, d + m(p2q2) + 0.5(1 + m)(p1q1 - p2q2) - v, where v is the cost to the pharmacist of the intervention. The return from a transfer will exceed the receipt from not intervening if:
(2)
or
(3)
For a proactive intervention made at cost vt in period, t, to the pharmacist over T periods and which causes proportion α < 1 of prescriptions to be written for drug 2 instead of drug 1, there is an incentive to pursue the intervention if, in each period, v < 0.5 (1 - m)(p1q1 - p2q2) as above, or if the discounted present value of returns to intervening with a given discount factor rt, that is,
(4)
exceeds the discounted present value of returns to not intervening,
(5)
We assume the net return to intervening is non-negative, that is,
(6)
although the pharmacist's optimal choice of effort (and hence á and v) might be low given a binding time constraint. This sufficient condition for participation is assumed to hold, at least weakly, for the pilot project since the pharmacist's discount factor for future returns and v are unknown.
Note that a, the proportion of prescriptions that are changed from drug 1 to drug 2, is not under the complete control of the pharmacist. An observation of low a, possibly the result of a low level of effort from the pharmacist due to the presence of higher returns to alternate activities, could also arise from a low level of response by physicians, a low proportion of patients eligible to be switched, or a high cost to the pharmacist of inducing a response.
Data description
The data cover the period from January 1993 to July 1998 and include specific information on each prescription dispensed, on a claim-by-claim basis, by the target pharmacy and three nearby pharmacies. The data are taken from two distinct sources: one is a provincial "Medicare"-type program and the other is a provincial prescription drug program. The database includes the dispensing date, the dispensing pharmacy, the prescribing physician, the drug, the dosage and quantity dispensed, record codes for each prescription reimbursed by the third-party insurer, and an intervention code. The pilot project involves one pharmacy, a clinic of eleven physicians, and three types of drugs (i.e., serum- lipid-reducing agents, agents acting on the renin-angiotensin system, and other anti-asthmatics, inhalants, glucocorticoids). For convenience, we refer to these groups as target pharmacy, target physicians, and target drugs. All others are referred to as non-target groups. To estimate cost savings due to pro-active interventions, we removed the effects of reactive interventions by eliminating all records that involve prescription interventions by the target pharmacy. We do not need to estimate cost savings due to reactive interventions as they are recorded electronically by the target pharmacy. Total drug expenditures are the sums of expenditures for remaining claims, on a monthly basis.
The study variable is the total monthly drug expenditures from January 1993 to July 1998. The data covers the 12 months of the intervention period, from August 1997 to July 1998, and the pre-intervention period, with 55 observations, starting January 1993.
Procedure
To estimate cost savings from pro-active intervention activities, we employ counter-factual analysis. Following this approach, we construct estimates, based on pre-intervention data, of total drug expenditures in the absence of interventions and compared these estimates to actual expenditures with the interventions under the pilot project. The approach consists of the following steps: (1) choosing an appropriate time-series forecasting model; (2) estimating and evaluating the model using the pre-event data; (3) using the model to form projections for the intervention period, which provides estimates in the absence of interventions; and (4) deriving cost savings from comparing the estimated drug expenditures to actual data in the intervention period. Of the four steps, the first step is the most crucial since a good estimate of cost savings will depend on a good forecasting model. Based on ex post forecasting accuracies as measured by the root mean square error, we selected the double exponential smoothing model with additive seasonal adjustment for estimating drug expenditures in the absence of interventions (i.e., counter-factual) during the intervention period.
Results
Trend analysis
To study the behaviour of the total drug costs for the target group, it is necessary to look at the trend patterns of the total drug costs in the entire target area during the sample period. A graphical inspection shows that first, the total drug costs in the target area follow a quadratic trend during the period of 1993 to 1998 with a peak occurring during the 1995-96 period and a downward trend over the remaining sample period. However, the total drug expenditures for the target and non-target groups show different trend patterns. In particular, while the total drug costs for the target area follow a downward trend since mid-1995, in terms of market share the target pharmacy has been gaining and the non-target pharmacies have been losing. Thus, if the interventions under the pilot were effective, we would expect to see increasing cost savings over time from this pharmacy. Second, with respect to target and non-target drugs groups, the total costs of the target drugs for all pharmacies exhibit an upward trend during the entire sample period, but the total costs of the non-target drugs follow a downward trend since mid-1995, indicating that, in the interest of cost savings, the pharmacy and the third-party insurer have chosen the right class of drugs to target. If the interventions were effective, we would likely see increasing cost savings over time from interventions focussed on the prescribing of these drugs. Finally, with respect to target versus non-target physicians, the total costs of drugs prescribed by target physicians and by non-target physicians follow exactly the opposite trends during the sample period, with target physicians gaining and non-target physicians losing their market shares. This implies that if the interventions were effective, we would also likely see an increasing cost savings over the intervention period. Hence, the right target has been "selected" in this pilot for reducing drug costs.
Primary results
This section presents the analysis of total drug costs for the target pharmacy-drug-physician group (i.e.,
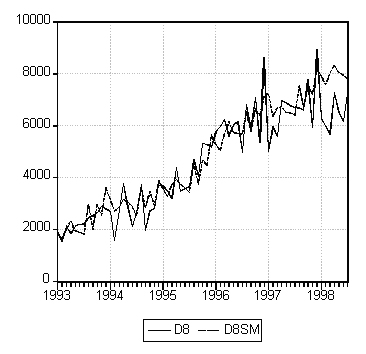
D8). Figure 1 shows that the forecasting series (D8SM) and the actual series (D8) trace each other very closely prior to the intervention period and there is an apparent difference between the two during the intervention period, especially during the second half of the period. The average mean difference is 16%. It might appear from this result that the intervention resulted in an average monthly savings of 16% during the twelve-month period. However, comparing the total costs of target drugs prescribed by the target physicians and dispensed by the target pharmacy (D8) with the total costs of target drugs prescribed by the target physicians and dispensed by other pharmacies, we find that the two series follow almost identical cyclical patterns during the intervention period. This admits to two contradictory explanations: either the intervention was so effective that it changed the behaviour of the target physicians in prescribing the target drugs and this effect spilled over completely to the prescribing of target drugs by non-target physicians; or the intervention had no effect at all and the apparent savings are due to some other factors such as the number of clients in the target group (C8). A simple regression of the total drug costs (D8) against C8 shows that over 92% of the variation in D8 is explained by the variation in C8.
Figure 1: Actual and estimated values of D8.
As an additional analysis, we used this model to project the total drug costs for the intervention period using the pre-event data. A t-test for equality of means between the actual series and the predicted one (or the counter-factual one) shows that there is no significant difference between the two series during the intervention period at the five percent level. Therefore, we conclude that there are no significant savings due to the intervention. The apparent saving seen in the aggregate data is due primarily to changes in the number of clients in that group. This conclusion is further strengthened by extending the analysis to include a dummy variable, which takes a value of 0 for the pre-event period and 1 for the intervention period in the regression equation. If interventions were effective, we would expect to see a negative and significant dummy coefficient. However, results show that the dummy coefficient is positive and significant at the five percent level. While this is consistent with our previous results, it does not explain why the total drug costs for the target group went up during the intervention period.
Additional results
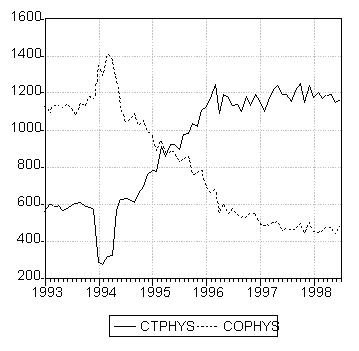
The preceding analysis indicates that there were no apparent savings from pro-active interventions under the pilot project during the period from August 1997 to July 1998. However, there were apparent shifts in other factors, such as the number of clients in each group. To capture this shift, the total numbers of clients of target versus non-target physicians are plotted in Figure 2 which reveals that the total number of clients of target physicians grew rapidly while that of non-target physicians fell sharply from the beginning of 1994 to early 1996. The numbers remained stable for the remaining sample period, from March 1996 to July 1998, with a slight upward trend for target physicians and downward trend for non-target physicians. The story being told here is that there was a shift in the market share of target versus non-target physicians and that pro-active interventions did not result in a decrease in the total number of patients with claims for target drugs.
Figure 2: Total number of clients of target physicians (CTPHYS) and other physicians (COPHYS).
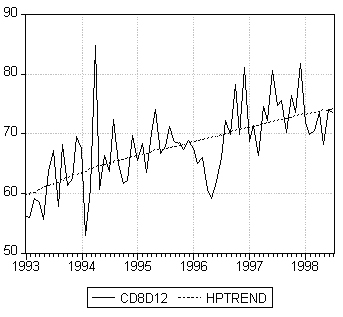
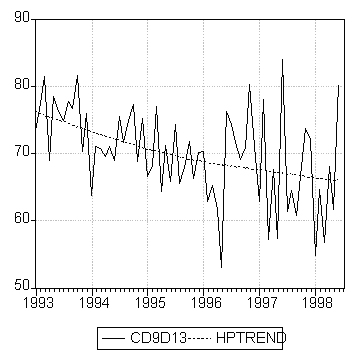
In light of this result, we further examine the trends in drug costs per client by target physicians, in Figure 3a, and by non-target physicians, in Figure 3b. The two series (CD8D12 for target and CD9D13 for non-target physicians) have opposite trends during the sample period with increasing drug costs per client by target physicians and decreasing drug costs per client by non-target physicians. For example, the average monthly drug costs per client by target physician during the intervention period were $73 compared to $66 by non-target physicians. The results further indicate that there was no evidence of cost savings from pro-active interventions in this pilot project.
Figure 3a: Drug costs per client, target physicians (CD8D12).
Figure 3b: Drug costs per client, other physicians (CD9D13
One type of target drug, namely serum-lipid-reducing agents, used to lower cholesterol, contains both high-cost products and low-cost alternatives that have identical effect. The high-cost products are being prescribed more often than the low-cost alternatives. Thus, it was expected that interventions for this type of drug would generate cost savings. We carried out statistical analysis for this single type of drugs following the same methodology as described above and found that there was a small level of cost savings, averaging $177 per month during the intervention period. However, it is statistically insignificant.
Conclusions
The central focus of this paper is the estimation of possible cost savings from the pro-active interventions in the pilot project. Trend analysis of the total drug expenditures reveals that, while the total drug costs for the target area (including all four pharmacies) follow a quadratic trend - rising and then falling over the sample period - the target and non-target groups follow opposite trend patterns with rising costs for the target and falling costs for the non-target groups. These trends arise from a shift in market shares over the intervention period: the target pharmacy and target physicians gained market share at the expense of the non-target pharmacies and the non-target physicians.
The estimation procedures demonstrate that while information on past expenditures is important in projecting future costs, relying on past expenditure information alone may yield misleading results, since regression analysis confirms that the number of clients is a strong predictor for the total drug costs. Including other variables, such as the prices of drugs, also improves the predicting power of the model.
The results show that although some savings can be achieved from reactive interventions (about 2 per cent), there are no significant savings from pro-active interventions. Any observed reduction in costs for the area stems primarily from a decrease in the number of clients. This reduction is not due to the pilot program.
Expenditures for one set of the target drugs, namely serum-lipid-reducing agents, containing both high-and low cost products, were studied following the same methodology. Estimates indicate that there is a small level of cost savings, averaging $177 per month during the intervention period. This result suggests that there may be potential savings from interventions into the prescribing of carefully selected drugs.
Further study, both of a longer experience with this project at the current site and of cross-sectional comparisons with projects at other sites, would be required to determine why this pilot project did not deliver significant cost savings in its current form. More information would be required to determine the cause of the results presented in this paper, in particular: a longer period over which to evaluate the pro-active interventions in the target area might uncover a delayed impact of invention efforts. Further, a second pilot site would allow some comparison of pharmacy and physician practices, with the possibility that the underlying causes of the results might become more apparent.
The question addressed by this assessment of the pilot project concerns the desirability of implementing a reimbursement model for pro-active interventions. The evidence does not lend extensive support for full implementation at this time.
Acknowledgments
We would like to thank two anonymous referees for their constructive comments. We have been the beneficiaries of the able research assistance provided by Jie Cui and Qiong Wu. This project was supported by a (provincial) government research contract. Opinions expressed in this paper are those of the authors and should not be construed as expressions of policy of any government, governmental agency, or its employees.
References
Law, S.M., Dickson, V.A., and Yu, W., Drug Cost Remediation and the Impact of Bill C-91, Federal/Provincial/Territorial Advisory Committee on Health Services, Ottawa, March 1997
Soumerai, S.M., Improving the quality and economy of in-hospital prescribing: getting more for less, Medical Journal of Australia, 149: 574-576, 1988.
The CPS Electronic Library [electronic resource], Login Brothers Canada, Winnipeg, 2001, formerly The Compendium of Pharmaceuticals and Specialties (CD-ROM), Login Brothers Canada, Winnipeg, 1999, formerly The Compendium of Pharmaceuticals and Specialties, Canadian Pharmaceutical Association, Toronto, 1974.
Anderson G. M., and Lexchin, J., Strategies for improving prescribing practice, Canadian Medical Association Journal, 154:1013-17, 1996.
Anis, A.H., Carruthers, G., Carter, A.O., and Kierulf, J., Variability in prescription drug utilization: issues for research, Canadian Medical Association Journal, 154:635-640, 1996.
Kolassa, E. M., Physicians' perceptions of prescription drug prices: their accuracy and effect on the prescribing decision, Journal of Research in Pharmaceutical Economics, 6(1): 23-37, 1995.
Ryan, M., Yule, B., Bond, C., and Taylor, R.J., Do physicians' perceptions of drug costs influence their prescribing?, PharmacoEconomics, 9(4): 321-331, 1996.
Schwartz, R.K., Soumerai, S.B., and Avorn, J., Physician motivations for nonscientific drug prescribing, Social Science Medicaid, 28(6): 577-582, 1989.
Coscelli, A., and Shum, M., How do doctors learn about new drugs? Evidence from panel data, Mimeo, Department of Economics, Stanford University, October 21, 1997.
Soumerai, S.B., and Avorn, J., Principles of educational outreach ('academic detailing') to improve clinical decision making, Journal of the American Medical Association, 263(4): 549-556, 1990.
Soumerai, S.B., and Avorn, J., Economic and policy analysis of university-based drug 'detailing', Medical Care, 24(4): 313-331, 1986.
Collins, T.M. , Mott, D.A., Bigelow, W.E., and Zimmerman, D.R., A controlled letter intervention to change prescribing behaviour: results of a dual-targeted approach, Health Services Research, 32(4): 471-487, 1997.
Ferber L.V., Luciano, A., Koster, I., and Krappweis, J., Drug utilization research in primary health care as exemplified by physicians' quality assessment groups, International Journal of Clinical Pharmacology, Therapy and Toxicology, 30(11): 453-455, 1992.
Santell, J.P., Projecting future drug expenditure – 1996, Working Paper, American Society of Health – System Centre on Pharmacy Practice Management, 1996.
Berman J.R., Zaran, F.K., and Rybak, M.J., Pharmacy-based antimicrobial-monitoring service, American Journal of Hospital Pharmacy, 49: 1701-1706, 1992.
Corresponding Author: Stephen Law, Department of Economics, Mount Allison University, 144 Main Street, Sackville, New Brunswick, Canada. slaw@mta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps