J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(3):234-244, 2002
Formulation of enterosoluble microparticles for an acid labile protein.
Ahmed Kashif Alavi1, Emilio Squillante III
Pharmacy and Administrative Sciences, College of Pharmacy, St. John's University, New York, USAKetan A. Mehta
Röhm Pharma Polymers, Degussa Corporation, 2, Turner Place, Piscataway, New Jersey, USAReceived 14 April 2002, Revised 30 July 2002, Accepted 16 September 2002
PDF version
Abstract
Purpose: A microencapsulation method that preserves the activity of an acid labile protein was developed. Methods: Solvent evaporation technique that employed ICH class 2 and 3 solvents methanol and acetone, respectively to dissolve pH-sensitive Eudragit polymers was investigated. Total protein released and lactase activities were measured using the USP method A for enteric cores and optimized with respect to process parameters. RESULTS: The percentage yields and entrapment efficiencies were directly proportional to solid content. The mean percentage yield and entrapment efficiency of selected sample was 84 ± 0.9% and 88 ± 0.7%, respectively. The residual specific activity of lactase in the selected sample was 89% ± 0.8 with a net activity loss of 2 ± 0.28% and 4 ± 0.52% under ambient and stressed storage, respectively. Dibutyl sebacate levels, lower processing temperatures and lower processing speeds were influential in modulating enzyme activity. The most important formulation factor affecting lactase stability was Eudragit type, followed in decreasing order by processing temperature, processing speed, and solid percentage. CONCLUSIONS: Reliable control of lactase release was achieved by microencapsulating the enzyme with pH-sensitive Eudragit L and S enteric polymers using either acetone- or methanol-based solvent but lactase activity was preserved only in acetone-based formulations.
Introduction
Lactase-phlorizin hydrolase (LPH) or B-galactosidase, the enzyme present in the brush border of the proximal jejunum enterocytes, hydrolyses (1) dietary lactose (from milk and dairy products) to glucose and fructose. LPH deficiency (2) leads to commonly manifested Lactose Intolerance (LI) and the rarer metabolic disorders (GM1 Gangliosidosis and Krabbe's disease). According to the data (3) compiled by the National Institute of Diabetes and Digestive and Kidney Diseases the total number of Americans affected by LI is between 30 to 50 million, which includes 75 percent of all African Americans and American Indians and 90 percent of Asian Americans. The American Dietetic Association estimates (4) that more than 70% of human population is affected to some degree with LI. Several authors (5-9) have highlighted the extent and effects of LI. Symptoms (10,11) of LI include bloating, diarrhea, abdominal cramps, distention, and flatulence, and are secondary to maldigestion, malabsorption and fermentation of lactose producing gas, lactate and short chain fatty acids. These symptoms are purportedly controlled to variable degree with the use of commercial lactase supplements e.g. Lactaid containing lactase (12,13) from Aspergillus oryzae a fungal species. In vivo methods e.g. breath hydrogen greater than 20 ppm in intolerant subjects as well as in vitro methods (14) to measure lactose hydrolysis do exist, however the stability of oral lactase supplements in the gastrointestinal tract (GIT) is highly questionable because of the acidic pH and the presence of digestive enzymes in the proximal GIT. This apparent lack of concern stems from the fact that commercially available lactase is classified as a dietary supplement, hence does not receive the scrutiny of rigorous FDA efficacy studies that all true drug substances must undergo. At the time of writing this manuscript there are no commercially available enteric-coated lactase products. Enteric formulation of lactase is more rational than immediate release commercial lactase products since the former protects the acid labile enzyme from gastric pH and delivers it to its physiological site of action, the small intestine. Thus this study was undertaken to formulate stable lactase microparticles with enteric release properties using solvent evaporation microencapsulation technique. The present work also lays the groundwork for polymer mixtures whose release profiles may be tailored to suit various formulation requirements.
MATERIALS AND METHODS
Chemicals
Eudragit L 100, S 100 and RS PO were a gift from Röhm Pharma, NJ. Enzeco lactase was gifted by Enzyme Development Corporation, NY. Sucrose stearate was donated by Montello Incorporated, OK. o-nitrophenyl-b-D-galactoside (ONPG) substrate was purchased from Pierce. Sodium carbonate, hydrochloric acid, DBS, TEC and sodium hydroxide were purchased from Spectrum Chemicals. Lactaid (4500 ALU units of activity per caplet) from McNeil Corporation was purchased from neighborhood pharmacies.
Rationale for the Selection of Ingredients and Processes
Enzeco lactase was used as a substitute for LPH and was selected (15-21) due to its broad stability profile, acidic pH optima, and the fact that it is also derived from the fungus Aspergillus oryzae , the source of the commercial lactase product Lactaid. Solvent evaporation o/o (oil in oil) emulsification method (22-23) of microencapsulation was chosen over solvent extraction since the former yields more uniform particles. The method is more correctly referred to as o/o (oil in oil) instead of w/o (water in oil) since a polymeric solution in organic solvent is considered as oil in microencapsulation terminology. The water soluble nature of lactase precluded the use of the more common o/w (oil in water) solvent evaporation procedure which would have caused partitioning of lactase in the external aqueous phase resulting in little or no entrapment. Choice of solvent system was based on the following criterion - solubility of ingredients, human safety, and compatibility with lactase. Squillante et al (24) had encapsulated lactase by solvent evaporation process using methanol -acetone mixture as solvent system focusing on the characterization of the prepared microparticles. The present study focussed on the preservation of biological activity for the enzyme and process optimization. The International Conference on Harmonization (ICH) class 2 solvent methanol was hypothesized to have adversely affected lactase activity in previous studies. Only a limited number of solvents have been used in literature to dissolve Eudragit polymers and Acetone, an ICH class 3 solvent with a much lower toxic potential, was a viable alternative and was evaluated. Pure acetone did not dissolve Eudragit; however acetone with 2% water fitted the criterion well. Eudragit S 100 (a slower dissolving enteric polymer which releases the drug later in the GIT as compared to Eudragit L 100) and the non-enteric Eudragit RS PO were used as formulation alternatives. Liquid paraffin was used as the dispersion media or external phase. Sucrose stearate (ScSt) was used as droplet stabilizer since it localizes at the interface between dispersed phase and dispersion medium promoting dispersion and preventing flocculation. Sucrose stearate was chosen over the more common droplet stabilizer Magnesium stearate because our screening studies showed that the latter was incompatible with anionic polymers (attributed to reaction of magnesium ions with the -CHO carboxylic group of the polymer) and also formed a less smooth coating due to lower solubility. Dibutyl sebacate (DBS) and triethyl citrate (TEC) were employed as formulation modifiers to facilitate the formation of microparticles and to modify the release properties. Hexane was used to clean the microparticles since it removes liquid paraffin without affecting the integrity of the microparticles. (25-30)
FCC Assay (Lactase Activity Assay Using ONPG)
An end point enzymatic assay (31) using ONPG as synthetic substrate for lactase was used to estimate the lactase activity. Incubation of ONPG substrate with lactase at 37°C for 10 minutes leads to formation of o-nitrophenol (ONP) and its absorbance at 415 nm is measured in a Hewlett Packard 8452A spectrometer or with a BioRad microplate reader. The amount of ONP formed gives a quantitative estimate of the activity of lactase, which is measured in acid lactase units (ALU). One ALU of lactase activity is defined as the quantity of enzyme that will liberate 1mol of o-nitrophenol (ONP) per minute at 37°C at a pH of 4.5.
Protein Assay
Quantitative estimation of protein in the dissolution sample was done by UV absorption protein assay at 280 nm in quartz cuvettes. A standard curve was first established by running controls with standard protein solutions within the dissolution vessels and subjecting them the same Dissolution Procedure as outlined below (including pH shift and filtration replicating the conditions of the actual dissolution tests). Validation was done by taking equal quantities of lactase in different dissolution vessels followed by quantitative protein assay.
Preliminary Screening
The preliminary screening included the effect of dissolution media on activity of Lactaid and Enzeco lactase, and effect of formulation ingredients and solvents on activity of Enzeco lactase. Kjeldahl nitrogen estimation (indirect method) and modified Bradford method (24,32) as well as UV absorption assay (direct methods) were used to estimate the total amount of protein in Enzeco sample. For studying the effect of dissolution media Lactaid and Enzeco samples were exposed to different pH using 1 molar Mc Ilvaine buffers in USP II dissolution apparatus for 2 hours at 37°C followed by FCC assay for residual lactase activity after the treatment. The effect of change in pH was also studied. Physical mixtures of Enzeco lactase with formulation ingredients (DBS, TEC, liquid paraffin, Eudragit L 100, Eudragit S 100, Eudragit RS PO, sucrose stearate) were evaluated over different temperatures and times for residual lactase activity using FCC lactase assay. The effect of different solvents (acetone with 2% water, methanol and hexane) on Enzeco lactase residual activity over contact time of half-hour and three hours was also studied using FCC lactase assay. Screening experiments to study the extent of binding to the Millipore filter and glass were also conducted.
Formulation
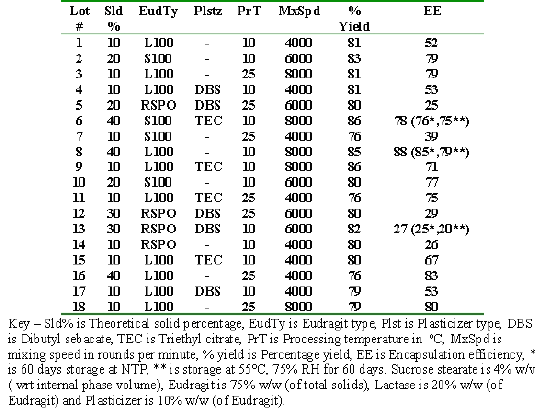
Modified solvent evaporation process (24) was employed (ingredients and conditions shown in table 1).
Table 1: Design of experiment, percentage yield, encapsulation efficiency and lactase activity.
Sucrose stearate and Enzeco lactase were suspended in 30 ml organic solution of Eudragit forming a coarse dispersion which was agitated with 200 ml liquid paraffin using a Brinkmann Polytron PT 3000 high shear homogenizer leading to the formation of emulsion droplets, which were stabilized with sucrose stearate. Low temperature, when required, was provided with an ice bath equipped with a thermometer. The gradual evaporation of the organic solvent effected over four hours led to nucleation and formation of microparticles which were washed five times with 50 ml of hexane, dried over vacuum for 12 hours and stored in dessicator. The percent yield of each of the samples was calculated from the expression
% Yield = (weight of microparticles / weight of solid starting material) X 100%
Selection of a working homogenizer speed range was done by operating it at variable speeds from 1000 rpm to 9000 rpm keeping other conditions and ingredients constant, followed by microscopic evaluation.
Experimental Design
The effects of Eudragit type, solid percentage, processing speeds, plasticizer type and processing temperature were studied. Results of screening studies were used to select the levels of sucrose stearate (4% w/v calculated with respect to internal organic phase volume), plasticizer (10% w/w calculated with respect to weight of polymer) and polymer to lactase ratio of 1:5. The experimental design was developed using S-matrix CARD software (S-matrix Corporation) with two levels of temperatures (10°C and 25°C), three levels of mixing speed (4000, 6000, and 8000), three levels of plasticizer (none, DBS, and TEC) and 4 levels of solid % (10, 20, 30 and 40). The 18 factor/level combinations of processing parameters chosen by the CARD software are shown in Table 1.
Dissolution Procedure
The USP Method A dissolution test for enteric cores (33) was performed in triplicate on the 18 formulations using a Van-Kel Model 6010 (Van-Kel Industries, Edison, NJ) at 37° ± 0.5°C and paddle speed of 50 rpm. Samples of various weights but all containing a theoretical equivalent of 450 mg of lactase (equivalent to 1 Lactaid tablet after taking the dilution factor into consideration) were added to 750 ml of N/10 hydrochloric acid solution (pH 1.2) which was the initial dissolution fluid for first 1 hour. Then a pH shift from 1.2 to 7.2 was effected by the addition of 107 ml of pH 7.2 phosphate buffer and 42 ml of 2 N NaOH. Sampling was done for an additional 1 hour at this pH. Dissolution samples were filtered through the same lot of Millipore 35 micron filters and assayed for lactase activity using the FCC procedure and for protein concentration using UV absorption as described above.
Entrapment Efficiency and Protein Estimation
The 18 different products were evaluated for entrapment efficiency calculated initially for three batches using the dissolution apparatus (involving a procedure similar to that described above under Dissolution Procedure ) and also by a much simpler and less cumbersome method using volumetric flasks described in detail below. Since the results obtained from both were in close agreement the latter method was adopted. Chilled pH 4.8 Mc Ilvaine buffer of constant ionic strength was taken in a 100 ml volumetric flask and to this was added accurate amount of microparticles (with theoretical equivalent to 10 mg lactase - calculated from the weight of individual ingredients and their proportion), quickly rinsed, filtered in a 25 mm Buchner funnel equipped with the same lot of VWRbrand Grade No. 417 coarse 40 micron (VWR Catalog 28313-080). Thus an estimate of `surface lactase' or the lactase not incorporated in the microparticles was made. In a second 100 ml volumetric flask pH 7.2 Mc Ilvaine buffer of constant ionic strength was taken, and accurately weighed microcapsules equivalent to 10 mg of lactase were added followed by agitation with a magnetic stirrer (Nuova II, Thermolyne) at maximum speed and an estimate of total lactase in the product was made. The difference between surface lactase and total lactase gives the amount of trapped or encapsulated lactase. The entrapment efficiency (EE) was calculated from the expression
EE = (Entrapped lactase / Initial lactase) X 100
Effect Of Formulation Ingredients on Activity
Schefe's model (A modified quadratic model without squared variables) was used by CARD Analysis of Data to analyze all the data obtained from activity assay. A regression model described the continuous smooth response surface graphs to visualize the combined linear and non-linear effects of the experimental variables on the activity. Optimization was also performed by the CARD software on the basis of the responses based on selection of conditions and/or parameters maximizing the biological activity of the enzyme.
Long Term Stability Studies
Long term stability studies were performed on samples stored under ambient room conditions for 60 days and compared with samples stored at 55°C and 75% RH in a saturated aqueous sodium chloride environment.
External Morphology, Particle Size and Size distribution
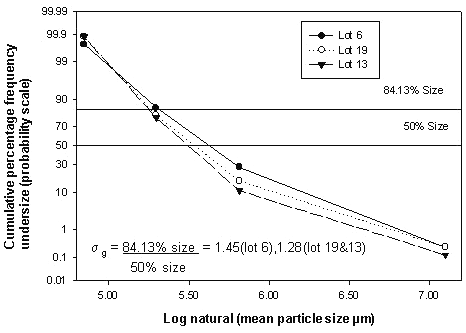
Gross morphology at 25 X magnification was studied by mounting the microparticles on clean glass slides and observing via light optical microscope for particle shape, clumping etc. SEM samples were coated with gold for 90 seconds under an argon atmosphere using a Polaron E 5100 coater at room temperature. A Jeol model 35 (Jeol, Peabody, MA) operated at a voltage of 25Kv and a working distance of 50 mm at an angle of 40° under 100 X and 300 X magnification (600 X for the final selected sample) was used to visualize the samples. Sieve analysis was used to estimate the geometric mean weight diameter (d') and the geometric standard deviation (s) as well as particle size distribution. 10, 40, 60, 100 and 140 mesh sieves were arranged with sieve 10 at the top, and sieve 140 at the bottom. A weighed sample was placed on the top sieve, shaken for 30 minutes, and the cumulative percent by weight of the powder retained on the sieve was plotted on the probability scale against the logarithm of the arithmetic mean sizes of two successive screens. The geometric mean weight diameter (d') was directly obtained from the graph by taking antilog of the 50% size while the geometric standard deviation (s) was calculated from the expression
s = 84.13% size/50% size
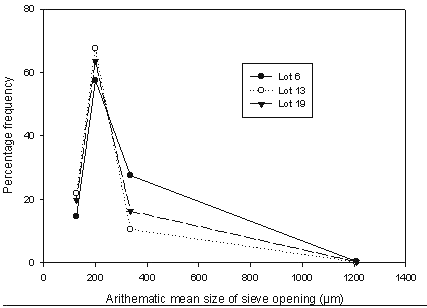
The size distribution was visualized by plotting the percentage frequency versus the particle size.
RESULTS
Table 2 shows effect of pH on the stability of Lactaid and Enzeco lactase.
Table 2: Percentage residual specific activity as a function of pH and time for Enzeco® lactase powder and Lactaid® chewable tablet.
Less than 5% loss in activity was seen at pH 7.2 but more than 90% of activity was lost at pH 1.2. The 90% of activity loss when pH was changed from 7.2 to pH 1.2 was not recovered when the pH was adjusted from 1.2 back to 7.2. However, there was no loss in activity when pH was changed from 7.2 to 4.5 and back to 7.2.
Enzeco sample was estimated to contain 40% protein using Kjeldahl nitrogen estimation method and modified Bradford method. All further work included this factor for all calculations of amount of protein. The percentage loss in activity on contact with unprocessed, physical mixtures of DBS, TEC, Eudragit L 100, S 100, RS PO, liquid paraffin and sucrose stearate was 1.42 ± 0.06, 1.47 ± 0.06, 2.41 ± 0.07, 2.22 ± 0.07, 2.61 ± 0.06, 3.39 ± 0.07, and 2.41 ± 0.07, respectively. These results were invariant with respect to changes in temperature or time. (p<0.05)
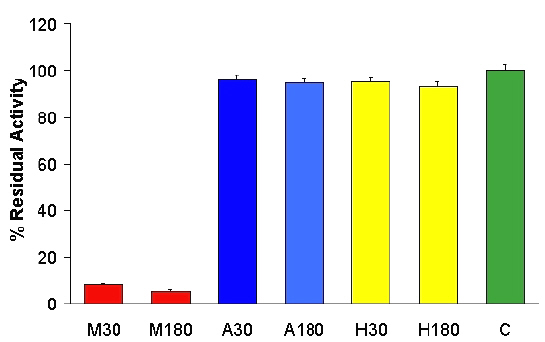
Contact with the strongly dipolar solvent methanol caused more than 90% loss in enzyme activity (shown in Figure 1), while the loss in activity for less polar solvents hexane and acetone was around 5%.
Figure 1: Effect of solvents - methanol, acetone (with 2% water) and hexane on lactase activity over a contact time of 30 minutes and 180 minutes.
The loss in activity in methanol was explained by its protein denaturant effect due to a high dielectric constant that causes disruption of the protein structure. The extent of binding to either the Millipore filters, VWRbrand filter paper, dissolution vessels, and disposable glass sample tubes showed the amount of lactase bound at concentrations from 0.3 to 0.9 mg/ml at pHs 1.2, 4.5 and 7.2 were always less than 1% of controls.(p<0.05)
In Table 3, speeds below 2000 rpm presented large, irregularly shaped particles (Figures 9) while speeds above 8000 rpm (Figures 10) yielded brown-tinted dispersions interpreted as visible signs of degradation.
Table 3: Effect of homogenizer speed on formation of microparticles.
SEMs of products processed between 4000 and 8000 rpm displayed uniform spheres and this range was selected for further investigation in the design of the experiment discussed earlier.
Percentage Yield and Entrapment Efficiency
The results are also shown in Table 1. The mean percentage yield for the 18 formulations was 81 ± 4%. A positive correlation between solid content and percentage yield was observed. This may be explained by the fact that though a constant amount of material is always lost in processing, this loss is proportionately less significant when the solid content is more (e.g. if the loss in processing is 100 mg then it more significant for a 1000 mg sample but much less significant for a 5000 mg sample.) Another reason for this effect is that the efficiency of the shearing process is increased with an increased solid content leading to an overall increase in the process efficiency and a better yield.
The mean entrapment efficiency (EE) was 60% ± 23.56. The large variation was due to the fact that Eudragit RSPO samples showed lower EE (25-29%) as compared to Eudragit S100 and L 100 (70-88%) samples. This may be explained by the fact that Eudragit RSPO swells and releases the drug in aqueous solutions irrespective of pH while Eudragit S100 and L 100 are enteric polymers and release drug only if pH is above 7 and 6 respectively. Higher EE was seen in samples formulated with a higher solid percentage (Lot 6, 8, and 16 had 40% solids) and an increase in processing temperature decreased the EE.
The mean percentage yield and entrapment efficiency of selected sample (Lot 19) was 84% ± 0.9 and 88% ± 0.7, respectively.
Effect of Formulation Ingredients on Activity
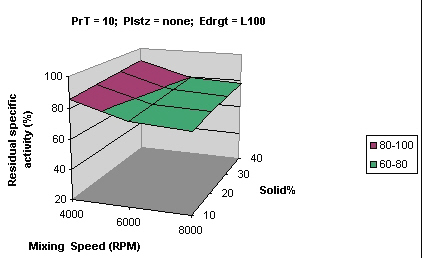
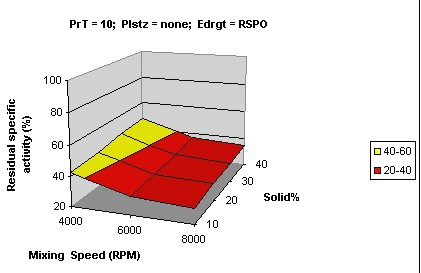
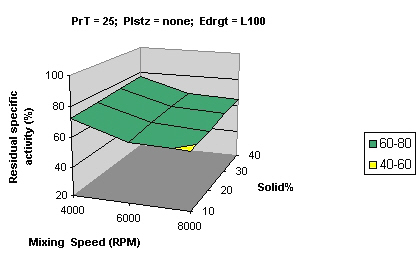
Figures 2 and 3 show that samples processed using Eudragit L 100 and S 100 had similar residual activity (> 80%) while samples processed with RS PO showed much lesser activity (20-40%).
Figure 2: Surface curve for mixing speed vs solid % for Eudragit® L 100 PrT processing temperature, Plstz Plasticizer, Edrgt Eudragit type.
Note - similar graph was obtained with Eudragit® S 100.
Figure 3: Surface curve for mixing speed vs. solid % for Eudragit® RS PO PrT processing temperature, Plstz Plasticizer, Edrgt Eudragit type.
This is due to the fact that Eudragit RS PO is not an enteric polymer and would not protect the enzyme.
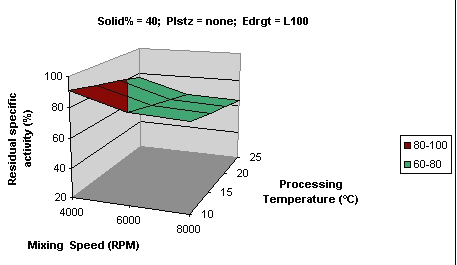
Comparison of Figure 2 with Figure 4 shows that lower temperature (10°C) had a protective effect on the activity even at higher mixing speeds, while higher temperature (25°C) coupled with higher speeds (8000 rpm) caused 40% loss in activity.
Figure 4: Surface curve for mixing speed vs. solid % for Eudragit® L 100 at 25°C PrT processing temperature, Plstz Plasticizer, Edrgt Eudragit type.
In general the formulations processed at higher temperatures had lower activity than the ones processed at lower temperatures. Also the formulations processed at higher speeds had lower activity than those processed at lower speeds which may be explained by the deleterious effect of shear forces and heat on enzyme activity.
Comparison between Figure 5 and Figure 2 shows that an increase in solid percentage also had a protective effect on the activity (increase by 5%) which may be explained by the dissipation of shear and heat in a larger amount of solid leading to lesser shear and heat strain per unit area.
Figure 5: Surface curve for mixing speed vs. processing temperature at 40% solids. Solid % percentage of solids, Plstz Plasticizer, Edrgt Eudragit type.
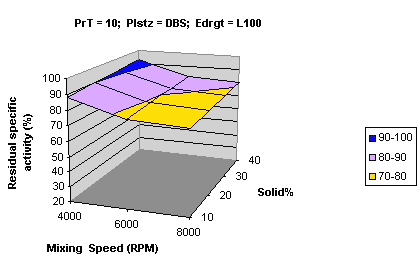
Figures 6-8 show the effect of plasticizers on activity and it was observed that both DBS and TEC had a protective effect on activity.
Figure 6: Surface curve for mixing speed vs. solid % using DBS at 10°C PrT processing temperature Plstz Plasticizer Edrgt Eudragit type.
Figure 7: Surface curve for mixing speed vs. solid % using TEC at 10°C. PrT processing temperature Plstz Plasticizer Edrgt Eudragit type.
Figure 8: Surface curve for mixing speed vs. solid % using TEC at 25°C PrT processing temperature Plstz Plasticizer Edrgt Eudragit type.
Figure 9: SEM (100x) showing effect of homogenizer speed 8000 rpm.
Figure 10: SEM (100x) showing effect of homogenizer speed 2000 rpm.
Figure 11: SEM of Lot 19 at 100 x.
Figure 12: SEM of Lot 19 at 600 x.
The protective effect of DBS was greater in magnitude. Another interesting observation was that for samples processed at 25°C using TEC, there was a loss of protective effect (Figure 8) while the protective effect of DBS was retained, and a graph similar to Figure 6 was obtained even at 25°C. The protective effect of the plasticizers was hypothesized to be due to the greater integrity and flexibility of the microparticles afforded by their plasticizing effect. A greater protective effect of DBS compared to TEC was hypothesized to be due to the fact that TEC is water soluble (~ 7%) unlike DBS making the formulations with TEC more prone to the degradative effect of the aqueous-acidic media leading to loss in the activity of the acid labile enzyme.
Long Term Stability Studies
Storage under stressed conditions (55°C and 75% RH) decreased the encapsulation efficiency. This was attributed to the deleterious effect of high moisture and temperature on the integrity of the microparticles.
The observed loss in activity under ambient storage for lot 19 sample was 2 ± 0.28% and under stressed storage was 4 ± 0.28% giving a 1 st order degradation constant of 1.01 X 10 -2 months -1 . The time required for 10% of the drug to degrade (t 10% ) was 11 months suggesting a reasonable shelf life for the product.
Figure 13: SEM of Lot 8 at 100 x.
Figure 14: SEM of Lot 8 at 300 x.
Figure 15: SEM of Lot 13 at 100 x.
External Morphology, Particle Size and Size distribution
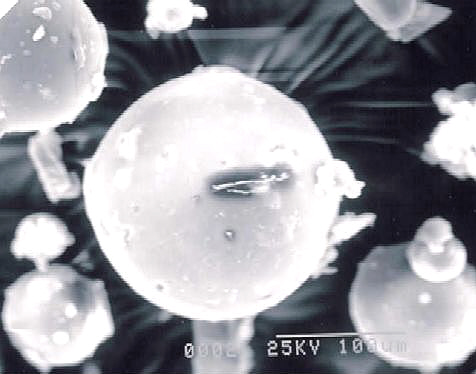
The scanning electron micrographs of the representative samples are shown in Figures 11-16.
Figure 16: SEM of Lot 13 at 300 x.
Figure 17: Plot of natural log diameter versus cumulative % frequency undersize for selected batches of microparticles.
Figure 18: Particle size-distribution curves for selected batches of microparticles.
Microparticles from the selected batch exhibited spherical morphology and smooth surface.
The geometric mean weight diameter was 195 ± 1.54 m for samples from Lot 6, 190 ± 1.28 mm for samples from Lot 13 and 195 ± 1.28 m from the final selected Lot 19 sample. The size and size distribution graphs for representative samples are shown in Figures 17 and 18.
Discussion
The results show that excellent EE and yields are obtained by either the methanol-based or acetone-based solvent evaporation processes, whereas enzyme activities were unacceptably low for lactase in the presence of methanol. Solvent evaporation microencapsulation technique was successfully modified to exclude the ICH Class 2 solvent methanol (which causes 90% reduction in lactase activity). Instead, these results show that a modified processing solvent of acetone (ICH class 3 solvent) with 2% water dissolved the ingredients without any deleterious effect on the stability of the enzyme. The percentage yield was directly proportional to solid content and was 81% for the final selected formulation. Minimum EE was seen with Eudragit RS PO samples while similar EE was seen with L 100 and S100 samples and EE was directly proportional to the percentage of solids and was inversely proportional to the processing temperature. For this reason, only acetone-based processes were considered a viable method of protecting lactase-containing products.
The most important formulation factor affecting the stability is the Eudragit type (L 100 samples had maximum, while RS PO samples had minimum activity), followed by temperature (10°C processing yielded a more stable product than 25°C processing), then speed (optimum speed was 4000), and then by solid percentage (optimum solid percentage was 40%). DBS was shown to have a preservative effect on stability. Higher speeds and processing temperatures had a detrimental effect on stability. The residual specific activity of lactase in the final selected product (Lot 19) was 89% ± 0.8 with a net activity loss of 2 ± 0.28% and 4±0.52% under ambient and stressed storage, respectively.
In summary, the deleterious and irreversible effect of pH on lactase activity observed in this report necessitates the need for the controlled release of enzyme in the GIT in a pH range conducive to enzyme activity. Using enzyme activity and release rate as criteria, we have demonstrated that lactase can be successfully formulated using enteric polymers under the optimal processing conditions of 10°C, 4000 rpm and 40% solids as microparticles that retain 88% residual specific activity and fulfill this stated objective.
This work is a portion of thesis work in a lab that is interested in stability of peptides and proteins and also conducts work on microdialysis and novel solvent mixtures like super critical fluids.
Acknowledgements
The authors acknowledge the grant provided by Röhm Pharma Polymers, Degussa Corporation, NJ. This work forms a part of the Master's thesis for Mr. Ahmed Kashif Alavi a graduate student at St.John's University, New York.
References
Quaroni, A. and Semenza, G., Partial amino acid sequences around the essential carboxylate in the active sites of the intestinal sucrase-isomaltase complex. J Biol Chem, 251:3250-3253, 1976.
Saavedra, J.M., and Perman J, A., Current concepts in lactose malabsorption and intolerance. Ann Rev Nutr, 9:475-502, 1989.
Lactose Intolerance, National Digestive Diseases Information Clearinghouse of National Institute of Diabetes and Digestive and Kidney Diseases, NIH Publication No. 02-2751, May 2002
Lactose Intolerance- Nutrition Fact Sheets of American Dietetic Association (ADA) , http://www.eatright.com/nfs/nfs43.html (accessed June 18, 2002)
Ryan, M.A., Living with lactose intolerance. Modern drug discovery, July/August, 87-91, 1999.
Hertzler, S.R., How much lactose is low lactose? J Am Dietetic Asso, 96:243-246, 1996.
Fermented Milks and Lactose Maldigestion, Danone World Newsletter, Nº12, August 1996. http://www.danonevitapole.com/nutri_views/newsletter/eng/news_12/sum.html (accessed June 18, 2002)
Calcium Counseling Resource-Lactose Maldigestion / Lactose Intolerance, National dairy council http://www.nationaldairycouncil.org/lvl04/nutrilib/calccounsel/06_ccr_rev.htm (accessed June 18, 2002)
Suarez, F.L., Savaiano,.D.A and Levitt, M.D., Review article: the treatment of lactose intolerance. Aliment Pharmacol Ther 9:589-597, 1995.
Bickerstaff, G.F., Industrial Applications, in Enzymes in Industry and Medicine, Edward Arnold Publishers, USA, 1990.
Ladas, S.D., Katsiyiannaki-Latoufi, E. and Raptis, S.A., Lactose maldigestion and milk intolerance in healthy Greek schoolchildren. Am J Clin Nutr, 53:676-680, 1991.
Gray, G.M., Congenital and adult intestinal lactase deficiency. N Engl J Med, 294:1055-1057,1976.
Literature, Enzyme Development Corporation, New York, 1998.
Zook, Christine M. and Lacourse, William R., Monitoring in vitro enzymatic digestion of lactose in milk using microdialysis with pulsed amperometric detection. Current Separations 17(2): 41-45, 1998.
Dey, P. M. and Pridham, J. B., Biochemistry of ß-galactosidase. Adv Enzymol.,36: 91-130, 1972.
Nevalainen, K.M.H., Induction, isolation, and characterization of Aspergillus niger mutant strains producing elevated levels of ß-galactosidase. Appl Environ Microbiol, 41:593-596, 1981.
Suckling,C.J., Gibson, C.I., and Pitt, A.R., Enzyme Chemistry: Impact and applications, 3rd edition., Blackie Academic and Professional, USA, 1998.
Dashevsky, A., Protein loss by the microencapsulation of an enzyme (lactase) in alginate beads. Int J Pharm, 161:1-5, 1998.
Chen, H., Langer, R., Oral particulate delivery: status and future trends. Adv Drug Delivery Rev, 34:339-350, 1998.
Allemann, E., Leroux, J., and Gurny, R., Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics. Adv Drug Delivery Rev, 34:171-189, 1998.
Thoma, K., Bechtold, K., Influence of aqueous coatings on the stability of enteric coated pellets and tablets, Eur J Pharm Biophar. 47:39-50, 1999.
Florence, A.T. and Jani, P.U., Particulate delivery: the challenge of the oral route, in Pharmaceutical Particulate Carriers- Therapeutic Applications, Rolland, A. (ed), Marcel Dekker, NY, USA, 1993.
Banga, A.K. , Therapeutic Peptides and Proteins., Technomic Publishing Company, Lancaster, 1995.
Squillante, E., Morshed, G., Mehta, K.A., Microencapsulation of ß-galactosidase with Eudragit L 100. J Micro, in press, 2002.
Lehmann, K.O.R., Chemistry and application Properties of Polymethacrylate Coating Systems. In Aqueous Polymeric Coatings for Pharmaceutical Dosage forms, 2nd ed., McGinity, J.W., (ed) Marcel Dekker, NY,USA, 1990.
Chern, C.S., Lee, .K., Chen, C.Y., Yeh, M.J., Characterization of pH-sensitive polymeric supports for selective precipitation of proteins, Colloids and Surfaces B. Biointerfaces, 6:37-49, 1996.
Thoma, K., Bechthold, K., Influence of aqueous coatings on the stability of enteric coated pellets and tablets, Eur J Pharm Biophar.47(1):39–50, 1999.
Lin, S, Liaob, C., Hsiueb, G., Liang, R., Study of a theophylline-Eudragit L mixture using a combined system of microscopic Fourier-transform infrared spectroscopy and differential scanning calorimetry, Thermochimica Acta.245:153-166, 1995.
Guoqiang, D, Batra, R., Kaul, R., Gupta, M.N., Mattiasson, B., 1995, Alternative modes of precipitation of Eudragit S 100: a potential ligand carrier for affinity precipitation of protein. Bioseparation.5:339-350.
Kumar, A. and Gupta, M. N., J Biotechnol, 37:185–189, 1994.
General Tests and Assays, Food Chemical Codex, FCC IV, 801-803, 1980.
Bradford, M.M., Anal Biochem., 72:248,1976.
Physical tests, Delayed Release (enteric coated) articles - general drug release standard, In USP 24, 1940-1947, 2000.
Corresponding Author: Ahmed Kashif Alavi, c/o E. Equillante, Room 105, St. Albert’s Hall, College of Pharmacy, St. John’s University, 8000, Utopia Parkway, Jamaica, New York, 11439, USA. squillae@stjohns.edu
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps