J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):123-130, 2002
Effect of the solvent system on the in vitro permeability of nicardipine hydrochloride through excised rat epidermis
Y.S.R. Krishnaiah1, V. Satyanarayana, R.S. Karthikeyan
Department of Pharmaceutical Sciences, College of Engineering, Andhra University, Visakhapatnam, A.P., IndiaReceived August 21 2001, Revised May 3, 2002, Accepted May 5, 2002
PDF version
Abstract
PURPOSE: The present investigation was carried out to study the effect of the solvent system on the permeation of nicardipine hydrochloride across excised rat epidermis in order to select a suitable solvent system for use in the development of a transdermal therapeutic system.
METHODS: The solubility of nicardipine hydrochloride in pure and mixed solvent systems was determined. The solvents used were water, propylene glycol, ethanol or various proportions of ethanol and water. The effect of these pure or mixed solvent systems on the skin permeation of nicardipine hydrochloride was also studied using in vitro permeation studies through excised rat epidermis mounted in modified Keshary-Chien diffusion cells. Fourier Transform Infrared Spectroscopy (FT-IR) and Differential Scanning Calorimetry (DSC) studies were carried out to study the effect of these solvents on the biophysical properties of rat' stratum corneum.
RESULTS: Although the solubility of nicardipine hydrochloride in propylene glycol (51.23 mg/mL) was higher than that in water (7.90 mg/mL) and ethanol (20.01 mg/mL), the flux of the drug from propylene glycol was as low (7.25±0.13 mg/cm2/h) as that from water (7.05±0.15 mg/cm2/h) and lower than that from ethanol (21.51±0.81 mg/cm2/h). The solubility of nicardipine hydrochloride in binary ethanol-water solvent systems in various proportions was higher than in pure solvents. The highest permeability was observed from an ethanol-water (70:30 v/v) solvent system (56.10±01.23 mg/cm2/h) which showed the highest solubility (224.21 mg/mL). The DSC and FT-IR data indicated that the binary solvent system containing ethanol-water in the ratio of 70:30 v/v increased the drug permeability through the skin by disrupting the highly ordered intercellular lipid structure of the stratum corneum in comparison with the untreated stratum corneum.
CONCLUSIONS: The results of the study indicate that the use of a binary solvent system, ethanol and water in the ratio of 70:30 v/v, is an effective vehicle for the development of a transdermal therapeutic system for nicardipine hydrochloride.
Introduction
Transdermal delivery of drugs may offer an attractive possibility because it avoids problems with gastrointestinal intolerance, reduces first-pass liver metabolism and removes the need to for intravenous access (1). Nicardipine hydrochloride is a calcium channel antagonist that possesses antihypertensive and arterial vasodilator properties (2). Although the drug is rapidly absorbed following oral administration, its bioavailability is relatively low due to first pass metabolism in the liver. Its biological half-life is very short (3) and is thus considered as an ideal drug candidate for transdermal drug delivery. The transdermal delivery of nicardipine hydrochloride depends on its permeability through stratum corneum, which in turn depends on the development of an optimal solvent system. Many approaches have been proposed in order to overcome the low permeability of drugs through the skin. They include organic solvents (ethanol, propylene glycol etc.) and fatty alcohols that can modify the skin structure and make channels in the skin barrier. Ethanol and propylene glycol are the most commonly used permeation enhancers that also act as cosolvents to solubilize drugs (4). Ethanol, used as part of a cosolvent system with water, has been demonstrated to increase penetration of a variety of drugs through the skin barrier (5-7). As a result, the use of cosolvents in the vehicle may exert a profound influence on the percutaneous delivery of drugs from topical dosage forms thereby providing a way to facilitate the desired penetration rate by adjusting the ratio of cosolvent used.
In the present study, the effect of several pure and mixed solvent systems on the in vitro skin permeability of nicardipine hydrochloride was studied in order to select a solvent system as the first step towards developing a transdermal therapeutic system.
Material and methods
Materials
Nicardipine hydrochloride was obtained from ICN Biomedicals, USA and acetonitrile (HPLC grade) was obtained from Qualigens Fine Chemicals, Mumbai, India. Triple distilled water (TD) was used. Other materials used in the study such as ethanol, propylene glycol and potassium dihydrogen phosphate were of analytical grade.
Methods
HPLC analysis of nicardipine hydrochloride
The quantitative determination of nicardipine hydrochloride was performed by High Performance Liquid Chromatography (HPLC). A Shimadzu Class VP series gradient HPLC with two LC-10AT VP pumps, an SPD-10A VP variable wavelength programmable UV/VIS detector, a CTO-10AS VP column oven, an SCL-10A VP system controller and a RP C-18 column (250 mm x 4.6 mm I.D.; particle size 5 mm; YMC, Inc., Wilmington, NC 28403, U.S.A) were used. The HPLC system was equipped with Class-VP series version 5.03 software.
The mobile phase consisted of a mixture of acetonitrile and 0.02M K 2 PO 4 (pH = 4.0) in the ratio of 60:40 v/v. The mobile phase components were filtered, and a flow rate of 1 mL/min was used for the separation. The column temperature was maintained at 40°C. The eluent was monitored at 239 nm, and the data were acquired, stored and analyzed with the previously mentioned software. A standard curve was constructed for nicardipine hydrochloride in the range of 0.01 to 2 mg/mL. A good linear relationship was observed between the concentration and area of nicardipine hydrochloride with a high correlation coefficient (r=0.9999). The required studies were carried out to estimate the precision and accuracy of this HPLC method of analysis for the quantitative determination of nicardipine hydrochloride. The standard curve constructed as described above was used for estimating nicardipine hydrochloride in the skin permeates.
Preparation of mixed solvent systems
Ethanol and water were mixed in different ratios to obtain binary solvent systems consisting of 25:75 v/v, 50:50 v/v or 70:30 v/v.
Solubility studies
Excess nicardipine hydrochloride was added to 10 millilitres of pure or mixed solvent system and vortexed. The mixture was immersed in a water bath at 37°C and allowed to equilibrate. The samples of 0.5 mL were obtained as function of time (12 h, 24 h and 36 h) and filtered through a 0.4- mm membrane filter, the filtrate was suitably diluted and the concentrations of nicardipine hydrochloride were estimated by the HPLC method described above.
Preparation of rat abdominal skin
The animals used for the preparation of skin were male albino rats (150-200 g) obtained from Ghosh Enterprises, Kolkota, India. They were permitted free access to food and water until used for the study. Care of the rats was in accordance with institutional guidelines. The rats were euthaniased using carbon dioxide asphyxiation. Dorsal hair was removed with a clipper and full thickness skin was surgically removed from each rat. The epidermis was prepared by a heat separation technique (8). The entire abdominal skin was soaked in water at 60°C for 60 s, followed by careful removal of the epidermis. The epidermis was washed with water and used in the in vitro permeability studies.
In vitro skin permeability studies
Modified Keshary-Chien diffusion cells were used in the in vitro permeation studies. The epidermis prepared as above was mounted between the compartments of the diffusion cells with the stratum corneum facing the donor compartment. The effective diffusional area was 3.5 cm 2 . The volume of the receiver compartment was 24 mL. Nicardipine hydrochloride was added to various pure solvents and various mixed solvents (10 mg/mL). The resulting drug solution or suspension was added to the donor cell. Ethanol and water in the ratio of 70:30 v/v was added to the receiver cell in order to maintain sink conditions. The cells were maintained at 37±0.5°C by a magnetic stirrer with heater (Remi Equipments, Mumbai, India). The contents in the receiver compartment were stirred with a magnetic bar at 500 rpm. At predetermined times (1, 2, 4, 6, 12 and 24 h) 0.5 mL samples were withdrawn from the receiver compartment and replaced with an equivalent quantity of drug-free solvent (70:30 v/v ethanol-water) to maintain a constant volume. The samples were assayed for nicardipine hydrochloride by HPLC as previously described.
Preparation of stratum corneum
The epidermis was incubated for 4 h in a 1% trypsin solution in phosphate buffered saline (pH 7.4) at 37°C. The tissue was then smoothed out on a flat surface and the mushy epidermis was removed by rubbing with a moistened-cotton-tipped applicator. The transparent stratum corneum obtained was briefly floated on water, blotted dry and used in the DSC (9) and FT-IR studies (10).
FT-IR Spectroscopy
The rat stratum corneum was treated with the relevant solvent systems for 24 h. The treated stratum corneum samples were vacuum-dried (650 mm of Hg) at 21± 1°C for two days and stored in a desiccators to remove traces of solvent (11). The completely dried samples of stratum corneum were then studied by FT-IR (Shimadzu, Japan). Attention was focused on characterizing the occurrence of peaks near 2851 cm -1 and 2920 cm -1, which were due to symmetric and asymmetric C-H stretching absorbances respectively.
DSC studies on stratum corneum
The rat stratum corneum was treated with the relevant solvent systems for 24 h. The treated stratum corneum samples were vacuum-dried (650 mm of Hg) at 21± 1°C for two days and stored in a dessiccator to remove traces of solvent (11). The changes in structure of stratum corneum were assessed by DSC (DSC 220C, Seiko Instruments, Inc. Japan) in terms of phase transition temperature of lipid components (9). The samples were scanned at 1°C/ min over the temperature range of 30-110°C.
Permeation data analysis and statistics
The nicardipine concentration was corrected for sampling effects according to the equation described by Hayton and Chen (12):
Where C 1 n is the corrected concentration of the n th sample, C n is the measured concentration of nicardipine hydrochloride in the n th sample, C n-1 is the measured concentration of the nicardipine hydrochloride in the ( n -1) Th sample, V T is the total volume of the receiver fluid and V S is the volume of the sample drawn.
The cumulative amount of nicardipine hydrochloride permeated per unit skin surface area was plotted against time, and the slope of the linear portion of the plot was estimated (13) as steady-state flux (mg/cm2/h).
The permeability coefficient (K p ) was calculated by using the following equation (14):
Where J SS is the steady state flux and C V is the initial concentration of nicardipine hydrochloride in the donor compartment.
The penetration enhancing effect of the solvent system was calculated in terms of enhancement ratio (ER) using the following equation (15):
Statistical comparisons were made using the student's t-test. A value of P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The solvent mixture design was employed to quantify its effect on nicardipine hydrochloride solubility in pure and mixed solvent systems (consisting of ethanol and water in various ratios). When the samples drawn at different time intervals were estimated for drug content, it was found that there was no further increase in the solubility of nicardipine hydrochloride after 24 hours. The results indicate that equilibrium was achieved at 24 h for nicardipine hydrochloride.
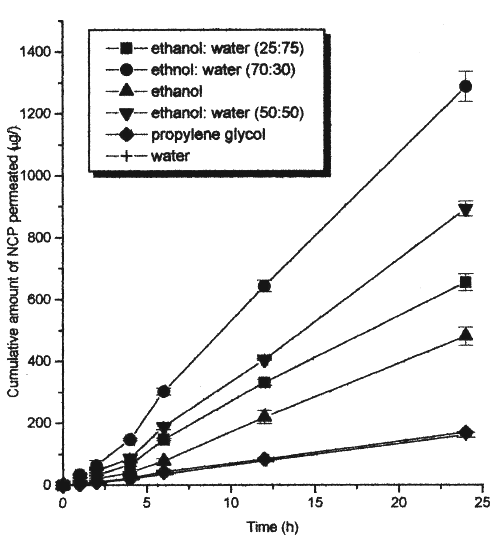
The solubility of nicardipine hydrochloride in various ratios of ethanol and water, and pure solvents is shown in Table 1. The solubility of nicardipine hydrochloride in propylene glycol, water and ethanol was 51.23 mg/mL, 7.90 mg/mL and 20.01 mg/mL respectively indicating a higher solubility in propylene glycol than that in water and ethanol. The solubility of the drug in binary ethanol-water systems 25:75 v/v, 50:50 v/v and 70:30 v/v was 89.56 mg/mL, 168.20 mg/mL and 224.21 mg/mL respectively. The solubility of nicardipine hydrochloride in several binary ethanol-water solvent systems was higher than that in both the pure solvents, which may be due to a cosolvency effect. The highest solubility in a binary system was observed in ethanol-water in the ratio of 70:30 v/v, which was approximately 10-20 times more than that in either water or ethanol alone. Figure 1 shows the skin permeation profile of nicardipine hydrochloride from pure water, ethanol, propylene glycol and binary solvent systems containing various ratios of ethanol and water.
Table 1: Solubility of nicardipine hydrochloride in various pure and mixed solvents at 37°C
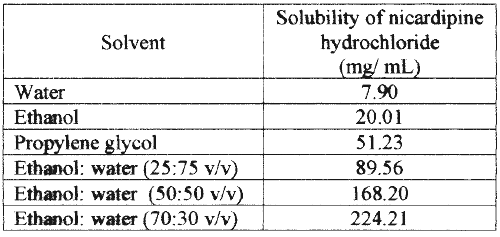
Figure 1: Mean (± s.d.) amount of nicardipine hydrochloride permeated from various solvent systems across the rat abdominal skin
The lag period for permeation of nicardipine hydrochloride through rat epidermis from all solvent systems was found to be 2 hours. The flux and permeability coefficients of nicardipine hydrochloride from various pure and mixed solvent systems are shown in Table 2. Although the solubility of nicardipine hydrochloride in propylene glycol was higher than that in other pure solvents, the skin permeation rate from propylene glycol was as low as that from water. The permeability flux of nicardipine hydrochloride in propylene glycol, water and ethanol was 7.25±0.13 mg/cm 2 /h, 7.05±0.15 mg/cm 2 /h, and 21.51±0.89 mg/cm 2 /h respectively.
Table 2: Mean (±s.d) flux (J), permeability coefficient (K p ) and enhancement ratio (ER) of nicardipine hydrochloride from various solvent systems (n=3)
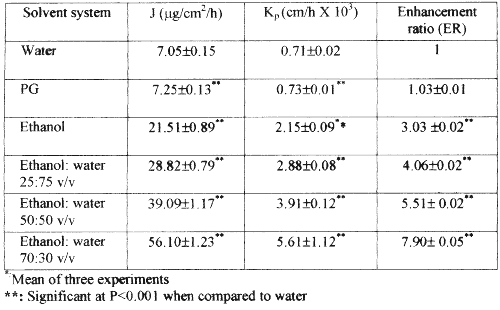
Skin permeation rates of nicardipine hydrochloride from ethanol were higher than that from water and propylene glycol solutions. In spite of its higher solubility in propylene glycol, nicardipine hydrochloride showed more or less the same permeability from both water and propylene glycol. This may be due to the similar thermodynamic activity of nicardipine hydrochloride in water and propylene glycol. The partitioning of the drug contained in propylene glycol from the donor compartment to the skin barrier phase appears to be low as evidenced by the low permeability coefficient (Table 2). If drugs are suspended in solvents and the solvents do not affect the skin barrier, the skin permeation rate of drugs should be constant irrespective of the nature of the solvent (16). On the other hand, skin permeation of nicardipine hydrochloride from ethanol was found to be higher than that from water and propylene glycol.
The skin permeation rate of nicardipine hydrochloride from the binary ethanol-water (70:30 v/v) mixture was found to be higher than that from the other binary solvent systems and from pure ethanol. The permeability flux for nicardipine hydrochloride in ethanol-water solvent systems in the ratio of 25:75 v/v, 50:50 v/v and 70:30 v/v was 28.82±0.79 mg/cm 2 /h, 39.09±1.17 mg/cm 2 /h and 56.10±1.23 mg/cm 2 /h respectively. This may be due to the varying influence of the ethanol-water solvent systems on the biophysical properties of the stratum corneum. However, the required flux of nicardipine hydrochloride was 116 mg/cm 2 /h which was calculated to maintain a therapeutic concentration of 24 ng/mL.
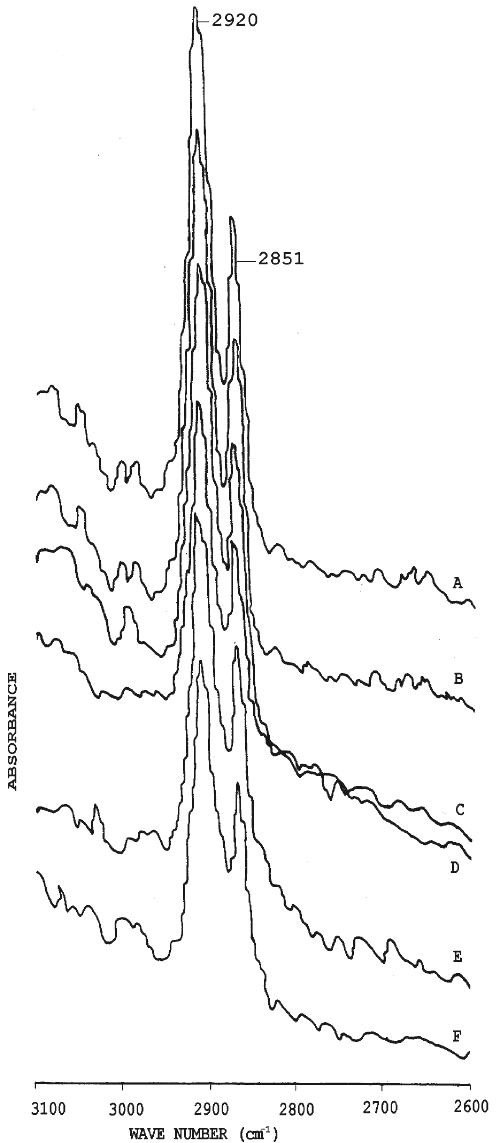
Figure 2: FT-IR spectra of stratum corneum of rat abdominal skin showing asymmetric and symmetric stretching absorbance after treatment with (A) water (B) propylene glycol (C) ethanol (D) 25:75v/v of ethanol-water (E) 50:50v/v of ethanol-water (F) 70:30v/v of ethanol-water
The FT-IR study provided an insight into the effect of the solvent systems on the biophysical properties of the stratum corneum. The treated stratum corneum was vacuum-dried, and was stored in a desiccator for the FT-IR studies (17-19). This is necessary to remove traces of solvents and to study the changes in the C-H stretching absorbance's caused by the particular solvent system. Figure 2 depicts the IR spectrum from 3100 cm -1 - 2600 cm -1 of the stratum corneum pretreated with different solvent systems. Table 3 shows the peak heights under the asymmetric and symmetric C-H stretching absorbances of rat's stratum corneum before and after treatment with various solvent systems used in the study.
Table 3: Mean (±s.d.) peak height of asymmetric and symmetric C-H stretching absorbances of rat stratum corneum lipids (n=3).
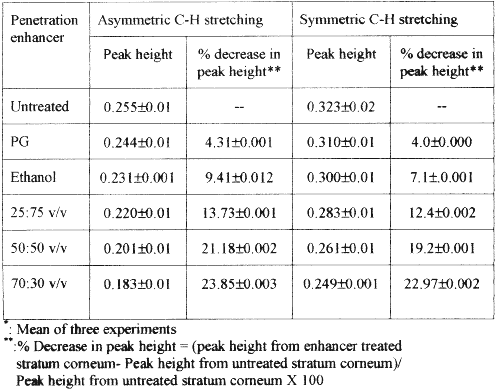
The lipid extraction resulting from the various solvents treatments was evaluated by comparing the intensities of the asymmetric and symmetric C-H stretching absorbances after treatment to those corresponding peaks before treatment.
The results of FT-IR showed that the treatment of stratum corneum with propylene glycol and various concentrations of ethanol did not produce a blue shift in the asymmetric and symmetric C-H stretching peak positions. However, they all showed a decrease in absorbance intensities for both asymmetric and symmetric C-H stretching when compared to the untreated stratum corneum. Ethanol and water in the ratio of 70:30 v/v produced a decrease in peak heights for asymmetric and symmetric C-H stretching absorbance by 22.97% and 23.85% respectively in comparison with untreated stratum corneum (Table 3). However, the percent decrease in peak heights for asymmetric and symmetric C-H stretching absorbance values with either pure ethanol, propylene glycol or other proportions of ethanol and water was very low. The significant decrease in peak height with 70:30 v/v ethanol-water suggests partial extraction of the lipids in the stratum corneum (20). There was no significant effect of propylene glycol on the extraction of lipids in rat's stratum corneum in comparison with the untreated stratum corneum.
Extraction of the lipids from stratum corneum leads to enhanced percutaneous absorption of drugs (21). Our findings suggest that greater extraction of the stratum corneum lipids by ethanol:water (70:30 v/v) led to greater permeability of nicardipine hydrochloride when compared to water, pure ethanol or other ethanol-water solvent systems. The increase in permeability of the drug may be due to increased solute diffusivity through the partially delipidised stratum corneum (22). Accordingly, the partially de-lipidised stratum corneum was highly permeable to the non-polar drug (nicardipine hydrochloride) used in this study.
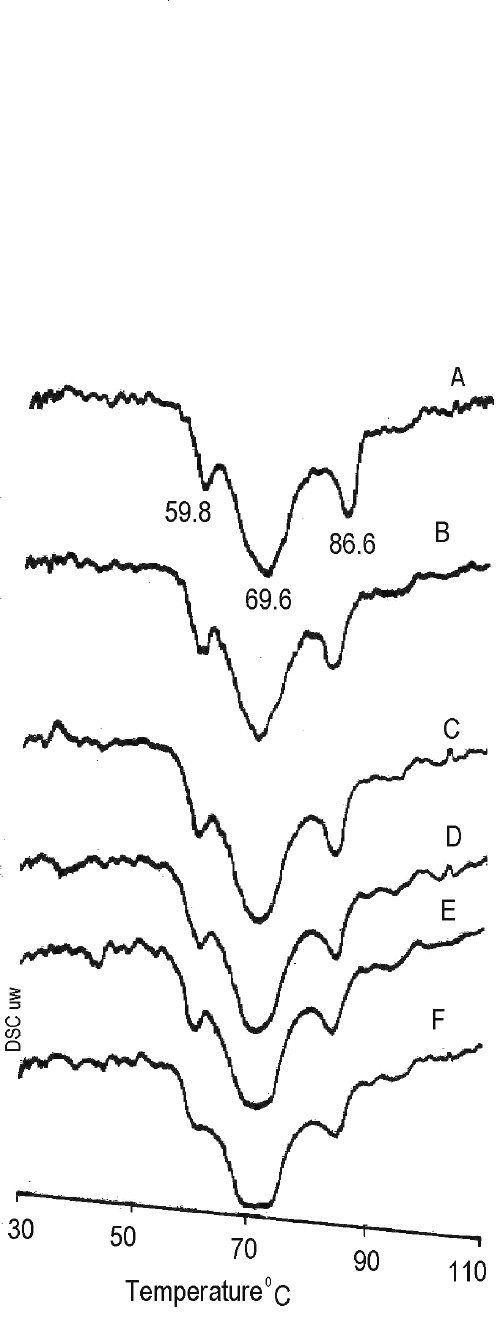
In order to obtain more detailed information of lipid components of the rat's stratum corneum treated with pure and mixed solvent systems, a DSC study was carried out. The DSC study is useful for characterizing the phase transition of the lipid bilayers, and conformational stability of the protein (23). The endothermic peaks obtained in the DSC study after treatment of rat's stratum corneum with different solvent systems are shown in Figure 3. An endothermic peak corresponding to the phase transition of constituent lipids was observed at 59.8°C, 69.6°C and 86.2°C in both solvent treated and untreated stratum corneum. However, there was a broadening of the endothermic peaks with the stratum corneum treated with 70:30 v/v ethanol: water and the 50:50 v/v ethanol: water solvent system. The results indicated that structures of lipid bilayers in the stratum corneum were slightly disrupted by treatment with ethanol and water in the ratio of 70:30 v/v. The increase in the permeability of nicardipine hydrochloride may be due to the disruption of stratum corneum lipid bilayers. The binary solvent system containing ethanol and water in the ratio of 70:30 v/v might be effective as a vehicle to enhance the skin permeation of nicardipine hydrochloride. Transdermal absorption of lipophilic drugs such as nicardipine hydrochloride, therefore, may be enhanced by using various solvents, which not only are able to solubilise drugs but also partially extract the lipids of stratum corneum in skin. Though the ethanol:water (70:30 v/v) solvent system provided good permeability of nicardipine hydrochloride, this may be insufficient to provide the required flux of the drug through the skin. Hence, further studies are in progress to study the effect of penetration enhancers like limonene, menthol and nerolidol on the permeability of nicardipine hydrochloride across excised rat epidermis.
Figure 3: DSC Thermograms of stratum corneum of rat abdominal skin after treatment with (A) water (B) propylene glycol (C) ethanol (D) 25:75v/v of ethanol-water (E) 50:50v/v of ethanol-water (F) 70:30v/v of ethanol-water
CONCLUSION
The present study was carried out to investigate the effect of solvent systems on the permeation of nicardipine hydrochloride across excised rat epidermis in order to select a suitable solvent system in the development of a transdermal therapeutic system for nicardipine hydrochloride. The solubility of nicardipine hydrochloride in pure and mixed solvents was studied and in vitro permeation studies for nicardipine hydrochloride from various pure and mixed solvent systems were carried out using excised rat epidermis. Although the solubility of nicardipine hydrochloride in propylne glycol (51.23 mg/mL) was higher than in water (7.90 mg/mL) and ethanol (20.01 mg/mL), the skin permeability rate of nicardipine hydrochloride from propylene glycol (7.25±0.13 mg/cm 2 /h) was as low as that from water (7.05±0.15 μ g/cm 2 /h) and lower than that from ethanol (21.51±0.81 mg/cm 2 /h). The solubility of nicardipine hydrochloride in binary ethanol-water solvent systems of various ratios was higher than that in pure solvents. The highest permeability (56.10±1.23 mg/cm 2 /h) was observed from an ethanol:water (70:30 v/v) solvent system, which gave the highest solubilty (224.21 mg/mL). The DSC and FT-IR data indicate that the binary solvent system of ethanol-water in the ratio of 70:30 v/v increases the drug permeability through the skin by partial extraction of the intercellular lipids in stratum corneum. The results of this study showed that the solvent system containing ethanol and water in the ratio of 70:30 v/v is suitable for use as a vehicle for developing a transdermal therapeutic system for nicardipine hydrochloride.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support received from the Government of India, Department of Science and Technology (DST) for supporting a research project under SERC scheme (Grant No: SP/SO/B70/97, dt. 28.1.1999). The financial support received from AICTE (MODROBS &TAPTEC) and UGC, New Delhi, India is greatly acknowledged in establishing the basic infrastructure needed for this study. The Authors greatly acknowledge Sipra Labs Pvt. Ltd., Hyderabad, India for FT-IR study.
References
- Bhatia, K. S., Goa, S., Freeman, T. P., and Singh, J., Effect of penetration enhancers and iontophoresis on the ultrastructure and cholecystokinin-8 permeability through porcine skin. J Pharm Sci, 86: 1011-1015, 1997.
- Graham, D.J.M., Dow, R.J., Hall, D.J., Alexander, O.F., Mroszczat, E.J. and Freedman, D., The metabolism and pharmacokinetics of nicardipine hydrochloride in man. Br J Clin Pharmacol, 20: 235-285, 1985.
- Dow, R.J. and Graham, D.J.M., A review of the human metabolism and pharmacokinetics of nicardipine hydrochloride. Br J Clin Pharmacol, 22: 1955-2025, 1986.
- Walker, R.B. and Smith, E.B., The role of percutaneous penetration enhancers. Adv Drug Deliv Rev, 18: 295-301, 1996.
- Obata, Y., Takayama, K., Maitani, Y., Machida, Y. and Nagai, T., Effect of ethanol on skin permeation of nonionised diclofenac. Int J Pharm, 89: 191-198, 1993.
- Berner, B., Otte, J.H., Mazzenga, G.C., Steffens, R.J.and Ebert, C.D., Ethanol: water mutually enhanced transdermal therapeutic system I: Nitroglycerin solution properties and membrane transport. J Pharm Sci, 78: 314-318, 1989.
- Takahashi, K., Tamagawa, S., Katagi, T., Yoshitomi, H., Kamada, A., Rytting, J., Nishihata, T. and Mizuno, N., In vitro transport of sodium diclofenac across rat abdominal skin: effect of selection of oleoginous component and the addition of alcohols to the skin. Chem Pharm Bull, 39: 154-158, 1991.
- Kaidi Zhao and Singh, J., Mechanism of percutaneous absorption of tamoxifen by terpenes: euginol, D-limonen and menthone. J Control Rel, 55: 253-260, 1998.
- Yui, N., Okuhara, M., Okano, M. and Sakurai, Y., Change in water structure in the stratum corneum of hairless rat skin by subcutaneous enhancers and its effect on indomethacin permeation. Jpn J Drug Deliv Syst, 7: 1199-1203, 1992.
- Bhatia, K.S., Gao, S. and Singh, J., Effect of penetration enhancers and iontophoresis on FT-IR spectroscopy and LHRH permeability through porcine skin. J Control Rel, 47: 81-89, 1997.
- Okamoto, H., Hashida, M. and Sezaki, H., Structure-activity relationship of 1-alkyl or 1-alkylzacycloalkanones derivatives as percutaneous penetration enhancers. J Pharm Sci, 77: 418-424, 1988.
- Hayton, W.L. and Chen, T., Correction of perfusate concentration samples removal. J Pharm Sci, 71: 820-821,1982.
- Julraht, K., Keith, A.P. and James, A.W., Development of a transdermal delivery device for melatoin in vitro studies. Drug Dev Ind Pharm, 21: 1377-1387, 1995.
- Scheuplein, R.J.; in: Jarret, A. (Ed.), The physiology and pathophysiology of skin, Vol. 5, Academic Press, New York, 1978.
- Williams, A.C. and Barry, B.W., Terpenes and the lipid-protein partitioning theory of skin penetration enhancement. Pharm Res, 8: 17-24, 1994.
- Higuchi, T., Physical analysis of percutaneous process from creams and ointments. J Soc Cosmetic Chem, 11: 85-93, 1960.
- Okamoto, H., Hashida, M. and Sezaki, H., Structure-activity relationship of 1-alkyl or 1-alkylzacycloalkanones derivatives as percutaneous penetration enhancers. J Pharm Sci, 77: 418-424, 1988.
- Yamune, M.A., Williams, A.C. and Barry, B.W., Effects of terpenes and oleic acid as skin penetration enhancer towards 5-fluorouracil as assessed with time; permeation, partitioning and Differential Scanning Calorimetry. Int J Pharm, 116: 237-251, 1995.
- Kurihara-Bergstrom, T., Knutson, K., DeNoble, L.J. and Goates, C.Y., percutaneous absorption enhancement of an ionic molecule by ethanol-water system in human skin. Pharm Res, 7: 762-766, 1990.
- Goates, C.Y. and Knutson, K., Enhanced permeation of polar compounds through human epidermis. I. Permeability and membrane structural changes in the presence of short chain alcohols. Biochem Biophys Acta, 1195: 169-179, 1994.
- Golden, G.M., Guzek, D.B.,.Harris, R.R., MeKie, J.E. and Pous, R.O., Lipid thermotropic transition in human stratum corneum. J Invest Dermatol, 86: 255-269, 1986.
- Yum, S.; Lee, E.; Taskovich, L.; Theeuwes, F., Permeation enhancement with ethanol: Mechanism of action through skin, In: D.S. Hsich (Ed.), drug permeation enhancement, Marcel Dekker, New York, 143-170, 1994
- Brandys, J.F., Hu, C.Q. and Lin, L., A simple model for proteins with interacting domains. Applications to scanning calorimetry data. Biochemistry, 28: 8588-8596, 1989.
Corresponding Author: Y.S.R. Krishnaiah, Associate Professor, Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam-530 003, India. krishnaysr112@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps