J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):162-168, 2002
Effect of powder polydispersity on aerosol generation
Nora Y.K. Chew, Hak-Kim Chan1
Faculty of Pharmacy, University of Sydney, Australia
Received 26 December 2001, Revised 3 June 02, Accepted June 10, 2002
PDF version
Abstract
Purpose. We investigated the effect of primary powder polydispersity on the generation of pharmaceutical powder aerosols, using mannitol and bovine serum albumin (BSA) as the model compounds.
Methods. Primary powders with different polydispersity but comparable physical and mass median aerodynamic diameter (MMAD) were obtained from spray drying. The polydispersity, i.e. the width of the particle size distribution, of the primary powder was measured by the span. Span = [particle diameter at 90% cumulative size] - [particle diameter at 10% cumulative size] / [particle diameter at 50% cumulative size]. A small span indicates a narrow size distribution. Solid state properties and the aerosol behaviour of the powders were determined.
Results. For both compounds, dispersion of the smaller span powder generated more fine particles below the MMAD of the primary powder, but the increase was less apparent at high air flows or with a high dispersion efficiency inhaler. With the Dinkihaler®, however, the smaller span powder of BSA, but not of mannitol, was found to experience less impaction loss and capsule and device retention. This could be due to the BSA powder being more cohesive, as it was amorphous and had a higher residual moisture content than that of mannitol.
Conclusions. The present study has demonstrated that the amount of fines available in the aerosol could be modified by the primary powder polydispersity.
Introduction
The impelling interest of pharmaceutical dry powder aerosols in recent years is driven by the urge to find a suitable replacement for the CFC-propellant liquid aerosols, as well as the recognition of the lung to deliver drugs systemically (1). Additionally, because of emerging technologies capable of making stable powders of respirable size, as well as devices competent to deliver flexible and accurate dosing, dry powder aerosols are no longer perceived as the "second choice" after the propellant driven liquid aerosols for inhaled drugs.
To advance powder aerosol technologies, scientists and manufacturers have recognised the importance of understanding the determinants affecting powder dispersion. The influences of particle surface characteristics (2-4), environmental conditions (5), air flow rate, (6), inhaler resistance (7-8), and excipients (9-10) on aerosol generation are some of the fundamental areas that have been under continuous investigation.
The importance of particle size on aerosol production has recently been demonstrated in our laboratory (11, 16). In those studies, primary powders, i.e., powders obtained from production such as spray drying of the same polydispersity but different particle sizes were prepared and dispersed at various air flows using inhalers of different air resistance. Our results showed that powder aerosol generation is an interplay of particle size, powder properties, air flow and inhaler design.
In contrast, the present study focuses on the effect of primary powders with different polydispersity on aerosol generation. Thus far, studies on aerosol polydispersity have been limited to lung deposition by mathematical modelling (12-15). These studies show that aerosols with the same primary particle size but different polydispersity will deposit differently in the lung. However this will only be true if the primary powder can be fully dispersed during aerosol generation.
The aim of the present study is to investigate the effect of powder polydispersity on the aerosol generation in vitro. The model compounds used in this study were mannitol and bovine serum albumin (BSA).
Materials and Methods
Powder Preparation
Primary powders with a similar mass median diameter (MMD) (~3 mm) (see next section for method of determination) but different polydispersity were obtained by spray drying (Büchi 191 Mini Spray Dryer, Flawil, Switzerland). The feed solution containing only the compound dissolved in deionised water was spray dried at conditions specified in Table 1. The spray-dried powders after collection were stored over silica gel or phosphorous pentoxide until use.
Table 1: Spray drying conditions for powders. (The aspiration rate was maintained at 57.6m3/hr except*, which was at 28.8 m3/hr)
Primary particle size distribution and Polydispersity
The particle size distribution of the primary powders was measured in suspensions using a Mastersizer S Laser Diffractometer (Malvern, Worcs, UK) as described previously (11, 16). Chloroform was used as a dispersion medium. The polydispersity of the powder was expressed by the span. Span = [D(v,90)-D(v,10)]/D(v,50), where D(v,90), D(v,10) and D(v,50) are the equivalent volume diameters at 90, 10 and 50% cumulative volume, respectively. The particle size of the primary powders was described by the volume median diameter (VMD), which is related to the mass median diameter (MMD) by the density of the particles (assuming a size independent density for the particles).
Powder dispersion
The method details have been given elsewhere (11, 16). Briefly, studies were conducted in an airtight perspex glove box temperature and relative humidity controlled at 23 ( ± 2)°C and 20 ( ± 5)% respectively. The dispersion behavior (the breaking up of agglomerates to regenerate the primary particles) of the spray dried powders was assessed by the Rotahaler® (Glaxo-SmithKline) and the Dinkihaler® (Aventis) coupled to a 4-stage (plus filter) liquid impinger (Copley, Nottingham, UK) with a glass throat. Capsule filled with 20 mg powders was used. Five capsules containing mannitol powder were dispersed per experiment, whereas four capsules were used for BSA.
Sample Assay
Mannitol was assayed by high performance liquid chromatography (HPLC) using refractive index detection. A 100 L aliquot was injected into a Waters (Milford, MA) HPLC system consisting of a 515 pump, a 410 refractive index detector and a 717 auto-injector. Data analysis was carried out using a Waters Millennium software (version 3.05.01). Mannitol was eluted from a Radial-Pak C18 reverse phase column (8mm id, 5 mm, Waters, Milford, MA) using a mobile phase containing deionized water only at a flow rate of 1.0 mL/min. The calibration curve of peak area versus mannitol concentration was linear in the concentration range of 0.40 - 2.00 mg/mL (R2 = 0.999, n = 10). BSA was assayed by UV spectrophotometry (Model U-2000, Hitachi, Tokyo, Japan) at 750 nm (Bio-Rad DC protein assay kit, Bio-Rad, Hercules, CA). The calibration curve was linear in the concentration range of 0.025 - 0.250 mg/mL (R2 = 0.998; n = 16).
Data Analysis
By definition, the primary powder after complete dispersion should have 50 wt.% of the powder with size below or above the mass median aerodynamic diameter (MMAD), which is calculated based on the product of the mass median diameter (MMD) of the primary powder and the square root of the true particle density. Since the two primary powders of each compound in this study have a similar MMD, they should give the same amount of fine particles after dispersion below the MMAD of the primary powder. This, in reality, is generally not true because of the incomplete dispersion of the powder. Here we define the relative fine particle fraction (rFPF) in the aerosol cloud as the amount of fine particles smaller than or with the same size as the MMAD of the primary powder. This is obtained by interpolation to the cumulative % undersize at the MMAD of each of the primary powder. It is referenced against the recovery (i.e. total dose = emitted dose + device and capsule retention) and is expressed as the means of three experiments.
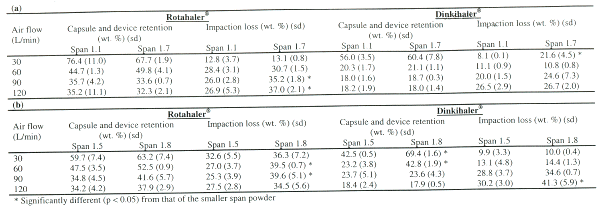
Impaction loss is the mass fraction of particles collected in the throat and stage 1 of the impinger. Capsule and device retention is the mass fraction of the particles collected at the capsule and device. Both the impaction loss and capsule and device retention were referenced against the recovery.
Data were subjected to analysis of variance (ANOVA) and Student's t-test (Microsoft Excel version 4.0), with probability values of less than 0.05 considered as statistically significant.
Powder characterization
Primary powder morphology - Powder samples were coated with platinum and examined under a Joel JSM 6000F scanning electron microscope (Tokyo, Japan).
Primary powder crystallinity - Samples packed on a glass sample plate under the storage humidity were analyzed on a Siemens D5000 X-ray powder diffractometer (Hamburg, Germany) using CuKa radiation generated at 40 kV and 30 mA, with an angular increment of 0.05°/ s.
Moisture content - Samples (~10 mg) were placed in platinum pans and heated at a rate of 5°/ min under a nitrogen purge (~30 mL/min) in a thermogravimetric analyzer (SDT 2960, TA Instruments, DE) controlled by a TA thermal solutions controller 4000. Percent moisture was calculated as the weight loss between room temperature and 130°C (for mannitol) or 200°C (for BSA) where the profiles leveled off.
True particle density - This was determined by a validated buoyancy method (11). Primary powder samples (1-2 mg) were placed in a density gradient liquid (comprising bromoform and 1-hexanol) and centrifuged (Jouan CT422, Saint Herblain Cedex, France) at 3500 rpm and 5°C for 30 min. The particle density was equal to the density of the liquid where the particles remained suspended after centrifugation.
Interior structure of particles - Freeze fracture was carried out for mannitol powders to examine the interior particle structure as described previously (11). The fracture surface was viewed under a transmission electron microscope (Philips 400, Eindhoven, The Netherlands) operating at 100 kV. For BSA, powder samples (approximately 1 - 2 mg) were fixed on a glass slide using glutaraldehyde (0.1 v/v%, Sigma, St. Louis, MO), dried overnight and viewed under a confocal microscope (Biorad Radiance Plus scanning system, Hertfordshire, UK) as described previously (4).
Results and Discussion
The present study investigated the role of the primary powder polydispersity on the generation of dry powder aerosols, using mannitol and BSA as model compounds. Mannitol and BSA powders were chosen for their different degree of cohesiveness, with BSA being more cohesive than mannitol as shown by their rFPF (Fig. 2 - 5).
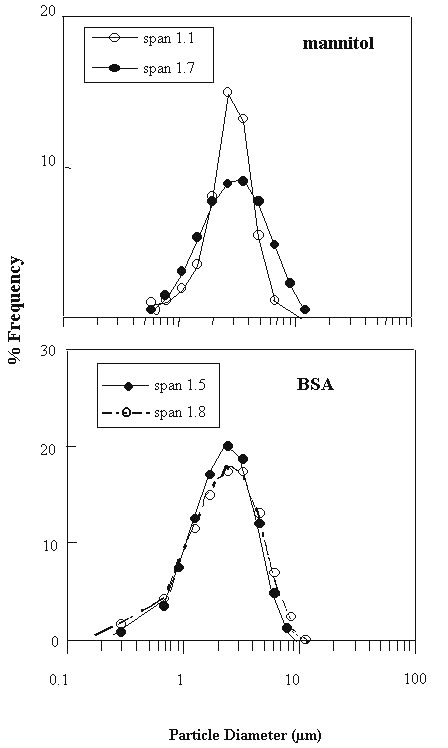
The "span" measures the width of the particle size distribution as described in the British Standards (17). Hence, a small span value indicates a narrow particle size distribution. For each compound, we have prepared two primary powders of comparable MMD (thus MMAD since both powders have the same true particle density) and particle morphology, but different spans. It has been demonstrated previously that the particle morphology is dependent on the nature of the compound, the spray drying process and the formulation variables (18, 19). In keeping the same particle morphology for the same compound, the two spans achieved were 1.5 and 1.8 for BSA, and 1.1 and 1.7 for mannitol (Fig. 1).
Figure 1: Primary Particle size distributions of mannitol and BSA powders
Table 2 summarizes the solid-state characteristics of these spray-dried powders. While both compounds yielded spherical non-hollow particles, BSA particles were amorphous with a higher residual moisture content, which presumably contributes to the cohesive nature of the powder.
Table 2: Spray-dried powders characteristics.
Since the two powders of each compound have a similar MMD, they should give the same rFPF after complete dispersion below the MMAD of the primary powder. In reality, the powders will not necessarily be fully dispersed due to the cohesiveness as commonly found for dry powder inhaler systems (20). This is indeed the case for BSA and a disparity was seen (discussed below). In contrast, mannitol being less cohesive (Fig. 2 & 3, comparing with Fig. 4 & 5 for BSA) is easier to disperse by the Dinkihaler® (11) and the two different span powders of mannitol did show a comparable rFPF when dispersed by the Dinkihaler®. At 30 L/min when the flow was not sufficient for powder de-agglomeration, a difference in the rFPF began to show up, with the larger span powder generating less fine particles in the aerosol. The same applied to the Rotahaler® which has a lower dispersion efficiency than the Dinkihaler®: a lower rFPF of mannitol was produced by the larger span powder (the opposite occurs at 120 L/min but the difference is not statistically significant with p = 0.07). For BSA, the difference in the rFPF between the two different span powders became even more obvious (Fig. 4 & 5). This is in concordance with the increased cohesiveness of the powders. As shown in Fig. 4, the span 1.8 powder has significantly lower rFPFs than the span 1.5 powder (p < 0.05, except at 120 L/min with the Rotahaler®) when dispersed at the same air flow.
The polydispersity of the primary powder affected the impaction loss. There was a greater proportion of larger particles present in the larger span powder, and these particles would have the potential to impact at the throat. Larger particles could also act as carriers with `binding sites' onto which the smaller particles adhere (20, 21), forming agglomerates for impaction. In comparison with the smaller span powder, the impaction loss for larger span powders was much higher and was flow-dependent, as impaction loss is proportional to the air flow and square of particle or agglomerate size (22).
Figure 2: Fine particle fraction of the mannitol powders dispersed by the Rotahaler® and the Dinkihaler®
Figure 3: Particle size distribution of the spray- dried mannitol powders before (without symbol) and after dispersion using the Rotahaler® and Dinkihaler® at different air flows.
Significantly higher (p < 0.05) impaction loss with the larger span powder was evidenced especially at high air flows (Table 3) for both mannitol and BSA. The polydispersity of the primary powder also affected capsule and device retention (Table 3). The effect tends to be more prominent at low air flows when there was not sufficient energy for powder emptying, as illustrated with the span 1.7 mannitol powder dispersed at 30 L/min using the Dinkihaler® (Table 3a), and with the span 1.8 BSA powder dispersed at 30 and 60 L/min (Table 3b).
Table 3: Capsule and device retention, and impaction loss from the dispersion of (a) mannitol, (b) BSA powders
Figure 4: Fine particle fraction of the BSA powders dispersed by the Rotahaler® and the Dinkihaler®
In conclusion, this study has shown that the primary powder polydispersity would affect the amount of fine particles (rFPF) generated in the aerosol clouds. Powders with a higher polydispersity have produced lower rFPF, higher impaction loss and higher capsule and device retention. Thus, for a given powder, a lower polydispersity could be chosen to optimize its aerosol performance. The effect is particularly significant when the powder is cohesive, or when a relatively low amount of energy (due to air flow or with a low dispersion efficiency inhaler) is available for powder dispersion.
Figure 5: Particle size distribution of the spray- dried BSA powders before (without symbol) and after dispersion using the Rotahaler® and Dinkihaler® at different air flows.
Acknowledgements
The authors would like to thank Ms Eleanor Kable for assistance in confocal microscopy and Mr. Tony Romeo for SEM; Drs Anna MacIntyre and Sandra Anderson for the availability of the Dinkihaler®. This work was supported by a grant from the Australian Research Council.
References
- Patton, J., Breathing life into protein drugs. Nat Biotechnol, 16: 141-143, 1998.
- Philip, V.A., Mehta, R.C., Mazumder, M.K., and DeLuca, P.P., Effect of surface treatment on the respirable fractions of PLGA microspheres formulated for dry powder inhalers. Int J Phar. 151: 165-174, 1997.
- Kawashima, Y., Serigano, T., Hino, T., Yamamoto, H., and Takeuchi, H., Design of inhalation dry powder of pranlukast hydrate to improve dispersibility by the surface modification with light anhydrous silicic acid (AEROSIL 200). Int J Pharm, 173: 243-251, 1998.
- Chew, N.Y.K., and Chan, H.-K., Use of Solid Corrugated Particles to Enhance Powder Aerosol Performance. Pharm Res 18: 1570-1577, 2001.
- Maggi, L., Bruni, R., and Conte, U., Influence of the moisture on the performance of a new dry powder inhaler. Int J Pharm, 177: 83-91, 1999.
- Smith, K.J., Chan, H.-K., and Brown, K. F., Influence of flow rate on aerosol particle size distributions from pressurized and breath-actuated inhalers. J Aerosol Med, 11: 231-245, 1998.
- Clark, A.R., and Hollingworth, A.M., The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers- implications for in vitro testing. J Aerosol Med. 6: 99-110, 1993.
- Srichana, T., Martin, G.P., and Marriott, C., Dry powder inhalers: The influence of device resistance and powder formulation on drug and lactose deposition in vitro. Eur J Pharm Sci 7: 73-80, 1998.
- Tee, S.K., Marriott, C., Zeng, X.M., and Martin, G.P., The use of different sugars as fine and coarse carriers for aerosolised salbutamol sulphate. Int J Pharm 208: 111-123, 2000.
- Chan, H.K., Clark, A., Gonda, I. Mumenthaler, M., Hsu, C., Spray dried powders and powder blends of recombinant human deoxyribonuclease (rhDNase) for aerosol delivery. Pharm Res, 14: 431-437, 1997.
- Chew, N.Y.K., and Chan, H.-K., Influence of particle size, air flow, and inhaler device on the dispersion of mannitol powders as aerosols. Pharm Res 16: 1098-103, 1999.
- Task Group on Lung Dynamics, Deposition and retention models for internal dosimetry of the human respiratory tract. Health Phys 12: 173-207, 1966.
- Gonda, I., A semi-empirical model of aerosol deposition in the human respiratory tract for mouth inhalation. J Pharm Pharmacol 33: 692-696, 1981.
- Gonda, I., Study of the effects of polydispersity of aerosols on regional deposition in the respiratory tract. J Pharm Pharmacol 33: 52P, 1981.
- Martonen, T., and Katz, I., Deposition patterns of polydisperse aerosols within human lungs. J Aerosol Med 6: 251-274, 1993.
- Chew, N.Y.K, Bagster, D.F., and Chan, H.-K., Effect of particle size, air flow and inhaler device on the aerosolisation of disodium cromoglycate powders. Int J Pharm 206: 75-83, 2000.
- British Standards #BS 2955, British Standards Institution, London, UK, 1993.
- Masters, K., Spray Drying Handbook. John Wiley & Sons, New York, 1976.
- Maa, Y.-F., Costantino, H.R., Nguyen, P.-A., and Hsu, C.C. The effect of operating and formulation variables on the morphology of spray-dried protein particles. Pharm Dev Technol 2: 213-223, 1997.
- Timsina, M.P., Martin, G.P., Marriott, C., Ganderton, D., and Yianneskis, M., Drug delivery to the respiratory tract using dry powder inhalers. Int J Pharm 101: 1-13, 1994.
- Staniforth, J. N., Rees, J. E., Lai, F. K., Hersey, J.A., Interparticle forces in binary and ternary ordered powder mixes. J Pharm Pharmacol 34: 141-145, 1982.
- Brain, J.D., Blanchard, J.D., Mechanisms of particle deposition and clearance, in F. Moren, M.B. Dolovich, M.T. Newhouse and S.P. Newman (eds) Aerosols in Medicine: Principles, Diagnosis and Therapy, Elsevier Science Publ.,
Corresponding Author: Hak-Kim Chan, Faculty of Pharmacy,Building A15, University of Sydney, NSW 2006, Australia. kimc@pharm.usyd.edu.au
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps