J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(2):199-204, 2002
Comparative efficacy and pharmacokinetics of racemic bupivacaine and S-bupivacaine in third molar surgery
J.P. Fawcett1, J.M. Kennedy, A. Kumar, R. Ledger
School of Pharmacy, University of Otago, Dunedin, New ZealandG.M. Kumara, M.J. Patel
Department of Stomatology, School of Dentistry, University of Otago, Dunedin, New ZealandM. Zacharias
Department of Anaesthesia and Intensive Care, Dunedin School of Medicine, University of Otago, Dunedin, New ZealandReceived 21 May, 2002, Revised 02 July 2002, Accepted 24 July 2002
PDF version
Abstract
Purpose: To compare the efficacy and pharmacokinetics of racemic bupivacaine (rac-bupivacaine) with S-bupivacaine as primary local anesthetic agent in bilateral impacted third molar extractions.
Method. A randomised, double blind, two period cross-over design was employed. Six subjects (2 males, 4 females; age 19-25 years; weight 69.2 ± 9.4 kg) received bupivacaine hydrochloride injection (6.6 ml) as rac-bupivacaine (0.5% as salt) or S-bupivacaine (0.5% as base) prior to extraction of impacted third molars on one side and three weeks later on the other side. Anesthesia, blood loss associated with surgery and post-operative pain experience were evaluated. Plasma samples were analysed for bupivacaine enantiomers by chiral HPLC.
Results. In 7/12 operations, anesthesia adequate for surgery was delayed (>10 min) or unsatisfactory requiring lidocaine rescue medication. Despite this, there were no significant differences in onset and duration of anesthesia, blood loss or post-operative pain experience between the two arms of the study. Pharmacokinetic parameters were not significantly different and there was no evidence of chiral inversion after dosing with S-bupivacaine.
Conclusions. Both study drugs were inadequate as single anesthetic agent for third molar surgery. Any decision to use S-bupivacaine for oral surgery must rest on evidence that it is less toxic than the racemic drug.
Introduction
Bupivacaine is an amide type local anesthetic widely used in surgery and obstetrics for sustained peripheral and central nerve blockade. It has a single chiral centre and is marketed as the racemate of R- and S-bupivacaine (rac-bupivacaine). Both enantiomers are active but S-bupivacaine produces a longer duration of neural blockade as well as a lower propensity towards central nervous system and cardiovascular toxicity (1-3). This has led to the introduction of S-bupivacaine into clinical practice under the name levobupivacaine (4). A parallel development has occurred for the propyl homolog of bupivacaine leading to the introduction of its S-enantiomer known as ropivacaine (5).
The safety and efficacy of S-bupivacaine has been compared with that of rac-bupivacaine in surgical anesthesia and pain management (4). In general, these studies have shown that S-bupivacaine has equivalent efficacy to that of rac-bupivacaine. The current practice in dental and oral surgical procedures is to administer lidocaine injection (2% lidocaine hydrochloride with epinephrine 1:100 000) prior to surgery and to use opiates or mild analgesics post-operatively. rac-Bupivacaine injection (Marcain®, 0.5% bupivacaine hydrochloride with epinephrine 1:200 000) has been used in patients undergoing removal of impacted third molars and shown to give a rapid onset time, good surgical anesthesia and few side effects (6-8). In comparison with lidocaine, bupivacaine provides an extended duration of post-operative analgesia and does not require the presence of epinephrine for consistent anesthesia (6). No information is available regarding the efficacy of S-bupivacaine in oral surgery.
This study was designed to compare pharmacodynamic and pharmacokinetic aspects of S-bupivacaine and rac-bupivacaine (both without epinephrine) when given as primary local anesthetic agent to subjects undergoing bilateral impacted third molar extractions. The fact that this type of surgery can be conveniently and beneficially performed on two occasions allows a two period cross-over design to be employed. The primary clinical endpoint of the study was time to onset of anesthesia adequate for surgery. Since efficacy in this regard depends on retaining the drug at the site of action, plasma concentration-time profiles of bupivacaine enantiomers were determined as an indication of relative retention at the site of action. In addition, chiral assay of bupivacaine enantiomers allowed an assessment of the stability of S-bupivacaine with respect to chiral inversion (conversion of S- to R-bupivacaine) in vivo. This is the first report of the use of S-bupivacaine in oral surgery.
Materials and Methods
Materials
rac-Bupivacaine hydrochloride injection (0.5% as the salt) and S-bupivacaine hydrochloride injection (0.5% as the free base) were supplied by Chiroscience Ltd. (Cambridge, England) in patient packs labelled according to a randomisation code. rac-Bupivacaine hydrochloride for use as a standard in assay development was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Samples of enantiomerically pure R- and S-bupivacaine hydrochloride were donated by Chiroscience Ltd. Lidocaine for clinical use was commercially available injection (2% lidocaine hydrochloride with epinephrine 1:100 000) purchased from Delta West (Bentley, Australia). Plasma for analytical development and validation was obtained from the Blood Bank, Dunedin Public Hospital. Heparinised Vacutainers® (10 ml) were purchased from Becton Dickinson (New Jersey, USA).
Subjects and clinical protocol
Subjects requiring surgical extraction of upper and lower third molars on both sides were recruited into the study. Based on previous clinical studies of bupivacaine in oral surgery (6-8), the time to onset of anesthesia was expected to be 2-10 minutes. To detect a clinically significant difference of 1 minute in time to onset of anesthesia at the 95% confidence level and with 90% power, 42 patients were required. Early in the study, it was found that the anesthesia achieved by the study drugs was either delayed or inadequate to allow surgery to proceed without recourse to lidocaine rescue medication. The trial was therefore terminated after six subjects had completed the protocol.
The six subjects (2 males, 4 females; age 19 to 25 years; weight 69.2 ± 9.4 kg) were healthy as determined by a full physical examination. The female subjects (A, C, E, F) were not pregnant or lactating mothers. All subjects gave written informed consent to participate in the study which was approved by the Southern Regional Health Authority Ethics Committee (Otago) and the Standing Committee on Therapeutic Trials, Ministry of Health, Wellington. All procedures were carried out according to the principles of Good Clinical Practice.
Each subject had surgical extraction of upper and lower third molars on one side on the first occasion and on the other side on the second occasion at least 3 weeks later. Subjects were given oral midazolam (15 mg) one hour before the administration of study drug. A venous cannula was inserted into the forearm for the collection of blood samples. Subjects then received a total dose of 6.6 ml of rac-bupivacaine (29.3 mg bupivacaine) or S-bupivacaine (33 mg bupivacaine) according to a randomisation schedule. Initially 4.4 ml of study drug solution was administered to the lower wisdom tooth site, of which 4 ml was used to block the inferior alveolar and lingual nerves and 0.4 ml to block the long buccal nerve. This was followed by 2.2 ml to the upper wisdom tooth site, of which 2 ml was infiltrated to the buccal side and 0.2 ml to the palatal side. After insertion of the needle for each administration, syringes were aspirated to check needles were not in blood vessels before delivery of study drug. For the purposes of blood sampling and pharmacodynamic assessment, time zero was deemed to be the start of administration of the study drug.
After drug administration, anesthesia of the lower jaw was monitored every 20 seconds by asking subjects whether their lip and tongue felt numb. One minute after reporting numbness, lower jaw anesthesia was assessed by gently pricking with a sharp dental probe between the lower premolar gum, the buccal gum and the lingual gum. Upper jaw anesthesia was assessed at the same time by gently pricking the gum on the buccal and palatal side of the upper third molar. Probing was continued at one minute intervals until all areas were declared as numb (onset of anesthesia). If numbness was not achieved within 10 minutes or if, during surgery, anesthesia was inadequate, lidocaine was administered by infiltration to the upper jaw, injection to the lower jaw or to both.
Once adequate anesthesia was achieved at both sites, extraction of the wisdom teeth was commenced. All blood, saliva and irrigating fluid used during surgery were collected and used to estimate blood loss by comparison of its hemoglobin concentration with the subject's whole blood hemoglobin concentration (9). The time from onset of anesthesia to when numbness of the lip and tongue receded as assessed by pricking was taken as the duration of anesthesia. The time from the start of numbness to when the subject first requested a dose of oral analgesic (20 ml of acetominophen 5%/codeine 0.15% elixir) was taken as the duration of analgesia. Post-operative analgesic consumption was based on the number of doses taken at four hourly intervals as required. Subjects assessed their perception of pain using a visual analog scale (VAS) at 15 minute intervals for the first hour and at various times thereafter. Blood samples (10 ml) were taken before and at specified times during surgery and for up to 6 hours thereafter.
All subjects were reviewed for adverse events the day after surgery and again 7 days post-surgery to remove sutures and for a clinical check as per normal practice. Three to four weeks after surgery, the same procedure was carried out on the other side prior to which subjects were given a dental examination and, for females, a pregnancy test. Any medication taken since the last visit was recorded.
Chiral analysis
Blood samples were centrifuged within 30 minutes of collection and plasma aspirated into plastic sample tubes and stored in a validated freezer at -35oC until analysis. Plasma samples (1 ml) were analysed for bupivacaine enantiomers by chiral HPLC after solvent extraction as previously described (10). The assay was linear over the range of 10-500 ng ml -1 of each enantiomer and coefficients of variation were in the range 1.5 to 15%.
Pharmacokinetic analysis
All concentrations are in ng ml -1 of the corresponding hydrochloride salts. Data are reported as mean ± standard deviation (s.d.) or mean ± standard error of the mean (s.e.m.). Maximum plasma concentrations of bupivacaine enantiomers (Cmax ) and the times to reach Cmax (tmax ) were noted directly from the data. The areas under the concentration versus time plots to infinity (AUCs) and the first moment curves (AUMCs) were determined by non-compartmental analysis based on statistical moments using Minim (version 3.08, Purves, 1993). Other parameters determined by non-compartmental analysis were: Total plasma clearance as CL = Dose/AUC; mean residence time (MRT) as AUMC/AUC; and terminal elimination half-life (t1/2β) as 0.693/b where is the elimination rate constant. ANOVA (general linear model) was used to compare pharmacokinetic parameters for R-bupivacaine and S-bupivacaine after administration of rac-bupivacaine and for S-bupivacaine after administration of S-bupivacaine and rac-bupivacaine. Statistical significance was set at P < 0.05.
RESULTS
Pharmacodynamic parameters for the twelve operations are given in Table 1.
Table 1: Pharmacodynamic parameters determined in six patients following injection/infiltration of rac-bupivacaine and S-bupivacaine for extraction of impacted third molars
In seven operations, onset of anesthesia was delayed (>10 min) and required lidocaine either before surgery could proceed or as rescue medication during surgery. One subject (C) required lidocaine in upper and lower jaw in both operations and had the slowest onset and shortest duration of anesthesia in both operations. Despite the use of lidocaine in some operations, there were no significant differences in the two arms of the study in onset and duration of anesthesia, blood loss associated with surgery, duration of analgesia, post-operative subjective assessment of pain (VAS scores) or post-operative analgesic consumption.
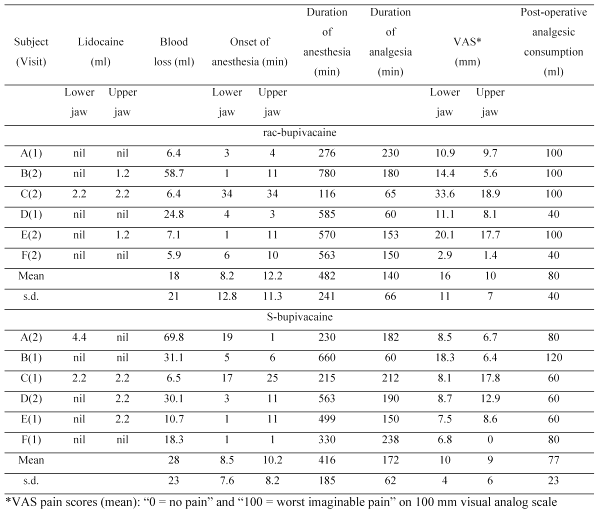
Mean plasma concentration-time profiles for R- and S-bupivacaine are shown in Figure 1 and pharmacokinetic parameters are given in Table 2.
Figure 1: oncentration-time profiles for R-bupivicaine [] and S-bupivacaine[] in plasma following injection/infiltration of (a) rac-bupivacaine and (b) S-bupivacaine to the same six patients in a cross-over design. Data are mean ± s.e.m.
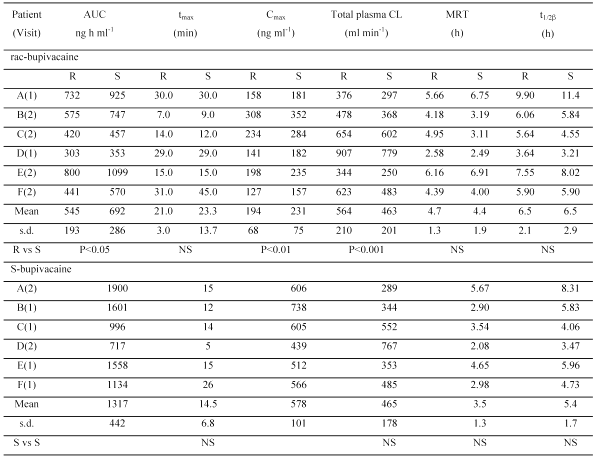
Table 2: Pharmacokinetic parameters determined in six patients following injection/infiltration of rac-bupivacaine and S-bupivacaine for extraction of impacted third molars.
After administration of rac-bupivacaine, tmax , MRT and t1/2β were not significantly different for the two enantiomers while AUC and Cmax were significantly greater for S-bupivacaine and CL was greater for R-bupivacaine. After administration of S-bupivacaine, there was no evidence of chiral inversion. The values of C max and AUC were greater than the corresponding sums of the values for R- and S-bupivacaine after administration of rac-bupivacaine but the differences were not significant. Values of CL and t1/2β for S-bupivacaine after administration of S-bupivacaine were not significantly different for S-bupivacaine after administration of rac-bupivacaine. Values of tmax and MRT were shorter but again the differences were not significant.
DISCUSSION
Previous workers have found onset of anesthesia after infiltration/injection of rac-bupivacine for oral surgery consistently occurred within two minutes and profound anesthesia was obtained in 4-10 minutes (7,11). In our hands, anesthesia adequate for surgery in both upper and lower jaw occurred rapidly (< 10 min) in only five operations although it occurred rapidly in either upper or lower jaw in another five operations. It has previously been hypothesized that accuracy of needle placement is a key factor in onset of anesthesia with bupivacaine because of its limited ability to diffuse from the site of injection through the nerve membrane (12). Whether this explains the need for lidocaine rescue medication experienced in our study is not clear particularly as other workers have observed that bupivacaine has a significantly longer onset time than lidocaine (13).
Studies of intradermal administration of R- and S-bupivacaine suggest that, compared to R-bupivacaine, S-bupivacaine has more vasoconstrictive activity at low concentrations and less vasodilator activity at high concentrations (14,15). On this basis, systemic absorption of S-bupivacaine should be slower than that of rac-bupivacaine with concomitantly greater local anesthetic activity. However, our study does not support this view since the tmax of S-bupivacaine tended to be shorter after administration of S-bupivacaine than after administration of rac-bupivacaine and there was no difference in blood loss associated with surgery for the two treatments.
The prolonged duration of anesthesia of 7-8 hours observed here is consistent with the findings of previous studies (7,16). However the duration of analgesia of 2-3 hours is shorter than the 5-7 hours typically reported in other studies. This may be because our protocol did not specifically instruct subjects not to take analgesics until they felt pain as done in other studies (8). Whatever the reason, our mean VAS pain scores of 9-16 (which largely reflect pain experience in the first hour after surgery) compare favourably with a value of 19 observed in a previous study of the same dose of rac-bupivacaine in third molar extractions (8). As regards a comparison of the study drugs with respect to post-operative pain experience, S-bupivacaine and rac-bupivacaine appear to have equivalent efficacy.
After administration of rac-bupivacaine, the higher AUC and Cmax and lower CL of S-bupivacaine relative to R-bupivacaine are consistent with the results of previous studies of intravenous infusion of rac-bupivacaine hydrochloride to healthy volunteers (17) and of injection/infiltration to subjects undergoing third molar extractions (8). The stereoselective pharmacokinetics of rac-bupivacaine has been attributed to the greater protein binding of S-bupivacaine (18). Pharmacokinetic parameters for S-bupivacaine after administration of S-bupivacaine and rac-bupivacaine were not significantly different consistent with a previous study in sheep (19). The stability of S-bupivacaine to chiral inversion in vivo is also consistent with previous studies (4). The AUC and Cmax of S-bupivacaine were somewhat higher than the sum of the values for R-and S-bupivacaine after administration of rac-bupivacaine consistent with the slightly higher dose employed. The similarity in elimination half-life of the study drugs provides further support to the premise that they are equivalent in terms of their retention at the site of action.
The highest blood concentration of bupivacaine hydrochloride in our study was 738 ng ml-1 (equivalent to 655 ng ml-1 of bupivacaine free base) after dosing with S-bupivacaine. This is below the lowest blood concentration of bupivacaine at which toxicity (a convulsive episode) has been reported of 1100 ng ml-1 (20). The highest concentration after dosing with rac-bupivacaine was 660 ng ml-1 (586 ng ml-1 of free base) which is much higher than that of 168 ng ml-1 observed by Danielsson et al after a dose of 44 mg rac-bupivacaine for third molar surgery (7). The reason for this marked difference is unclear although the possibility it results from the presence of epinephrine in the formulation used by Danielsson et al cannot be ruled out.
In conclusion, our study shows that neither rac-bupivacaine nor S-bupivacaine is suitable as primary anesthetic agent in third molar surgery. It also reveals that both study drugs are useful in providing sustained post-operative analgesia and that S-bupivacaine is not superior in this regard. These conclusions are unlikely to be compromised by the small population size of our study and are consistent with recent clinical studies which found no statistical difference in pharmacodynamic parameters between rac-bupivacaine and S-bupivacaine in surgical procedures and pain management (4). Thus it is clear that any decision to adopt the single enantiomer formulation of bupivacaine for oral surgery must be based on the evidence of its lower toxicity compared to the racemate.
ACKNOWLEDGMENTS
The authors are indebted to Anne Walker, Gabriel Sertsou and Dorothy Saville (School of Pharmacy, University of Otago, Dunedin) for helpful suggestions and to the staff of the Department of Stomatology, School of Dentistry, Dunedin for invaluable assistance. The authors gratefully acknowledge the sponsorship of this study by Chiroscience Ltd. (Cambridge, England).
REFERENCES
Mather, L.E., McCall, P. and McNicol, P.L., Bupivacaine enantiomer pharmacokinetics after intercostal neural blockade in liver transplant patients. Anesth Analg, 80:328-335, 1995.
Huang, Y.F., Pryor, M.E., Mather, L.E. and Veering, B.T., Cardiovascular and central nervous system effects of intravenous S-bupivacaine and bupivacaine in sheep. Anesth Analg, 86:797-804, 1998.
Gristwood, R.W., Cardiac and CNS toxicity of levobupivacaine: strengths of evidence for advantage over bupivacaine. Drug Saf, 25:153-163, 2002.
Foster, R.H. and Markham, A., Levobupivacaine A review of its pharmacology and use as a local anaesthetic. Drugs, 59:551-579, 2000.
McClellan, K.J. and Faulds, D., Ropivacaine: An update of its use in regional anesthesia. Drugs, 59:551-579, 2000.
Laskin, J.L., Wallace, W.R. and DeLeo, B., Use of bupivacaine hydrochloride in oral surgery - a clinical study. J Oral Surg, 35:25-29, 1977.
Danielsson, K., Evers, H., Holmlund, A., Kjellman, O., Nordenram, A. and Persson, N.E., Long-acting local anaesthetics in oral surgery. Int J Oral Maxillofac Surg, 15:119-126, 1986.
Bouloux, G.F. and Punnia-Moorthy, A., Bupivacaine versus lidocaine for third molar surgery: A double-blind, randomised, crossover study. J Oral Maxillofac Surg, 57:510-514, 1999.
Dacie, J.V.; Lewis, S.M., Practical haematology. 7th ed., Churchill Livingstone, New York, NY, USA, 1991.
Abraham, I., Fawcett, J.P., Kennedy, J., Kumar, A. and Ledger, R., Simultaneous analysis of lignocaine and bupivacaine enantiomers in plasma by high-performance liquid chromatography. J Chromatogr B, 703:203-208, 1997.
Pricco, D.F., An evaluation of bupivacaine for regional nerve block in oral surgery. J Oral Surg, 35:126-129, 1977.
Sisk, A.L., Long-acting local anesthetics in dentistry. Anesth Prog, 39:53-60, 1992.
Morgan, M. and Russell, W.J., An investigation in man into the relative potency of lignocaine, bupivacaine and etidocaine. Br J Anaesth, 47:586-591, 1975.
Luduena, F.P., Duration of local anesthesia. Ann Rev Pharmacol, 9: 503-520, 1969.
Aps, C. and Reynolds, F., An intradermal study of the local anaesthetic and vascular effects of the isomers of bupivacaine. Br J Clin Pharmacol, 6:63-68, 1978.
Moore, P.A. and Dunsky, J.L., Bupivacaine anesthesia – A clinical trial for endodontic therapy. Oral Surg, 55:176-179, 1983.
Burm, A.G.L., Van Der Meer, A.D., Van Kleef, J.W., Zeijlmans, P.W.M. and Groen, K., Pharmacokinetics of the enantiomers of bupivacaine following intravenous administration of the racemate. Br J Clin Pharmacol, 38:125-129, 1994.
Veering, B.T., Burm, A.G., Feyen, H.M., Olieman, W.M., Souverijn, J.H. and Van Kleef, J.W., Pharmacokinetics of bupivacaine during postoperative epidural infusion: Enantioselectivity and role of protein binding. Anesthiology, 96:1062-1069, 2002.
Mather, L.E., Huang, Y.F., Veering, B. and Pryor, M.E., Systemic and regional pharmacokinetics of S-bupivacaine and bupivacaine enantiomers in sheep. Anesth Analg, 86:805-811, 1988.
Hassellstrom, L.J. and Mogensen, T., Toxic reaction of bupivacaine at low plasma concentration. Anesthiology, 61:99-100, 1984.
Corresponding Author: J.Paul Fawcett, School of Pharmacy, University of Otago, P.O. Box 913, Dunedin, New Zealand, paul.fawcett@stonebow.otago.ac.nz
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps