J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 5(1):29-38, 2002
Influence of Phytostanol Phosphoryl Ascorbate, FM-VP4, on Pancreatic Lipase Activity and Cholesterol Accumulation within Caco-2 Cells.
Manisha Ramaswamy, Edwin Yau, Kishor M. Wasan1
Division of Pharmaceutics and Biopharmaceutics, Faculty of Pharmaceutical SciencesKathy D. Boulanger, Ming Li, P. Haydn Pritchard
Pharma Division, Forbes Medi-Tech Inc., Department of Pathology and Laboratory Medicine, University of British Columbia, B.C, CanadaReceived December 5, 2001, Revised April 24, 2002, Accepted April 25, 2002
PDF version
Abstract
Purpose: The objective of this study was to determine how a novel hydrophilic phytostanol (FM-VP4) affects the cellular accumulation of [ 3H]cholesterol in human colon carcinoma (Caco-2) cell monolayers grown in Transwell® chambers.
Methods: To determine cellular accumulation of cholesterol and FM-VP4, [ 3H]cholesterol- containing micelles (50 mM cholesterol containing 1.27x10 -4% [ 3H]cholesterol) or [ 3H]FM-VP4 (50 mM) was incubated on the apical side of differentiated Caco-2 cell monolayers for 1 to 4 h at 37°C in the absence or presence of increasing concentrations (10-200 mM) of unlabeled FM-VP4 or cholesterol, respectively.
Results: The accumulation of [ 3H]cholesterol (presented in micelles) into Caco-2 cell monolayers in the presence of 50 mM FM-VP4 was significantly lower (33.7 ± 7.0%) compared to control (59.8 ± 5.2%, p<0.05) following 4 h of incubation. Conversely, cholesterol inhibited the accumulation of [ 3H]FM-VP4, although to a lesser extent, suggesting competition for binding sites. The inhibitory effects of FM-VP4 and cholesterol on each other were detectable after 1 h of incubation and increased with time. The extent of FM-VP4 inhibition of [ 3H]cholesterol accumulation was consistent whether FM-VP4 was co-incorporated into micelles or added separately in solution, suggesting that FM-VP4 does not elicit its effects through inhibition of cholesterol incorporation into micelles. In addition, pancreatic lipase activity ([ 3H]triolein hydrolysis) and p-glycoprotein (rhodamine 123 fluorescence) activity, were not affected by FM-VP4.
Conclusions: In conclusion, FM-VP4 rapidly inhibits cholesterol accumulation within Caco-2 cell monolayers in a mode independent of pancreatic lipase activity, p-glycoprotein activity or cholesterol incorporation in micelles.
Introduction
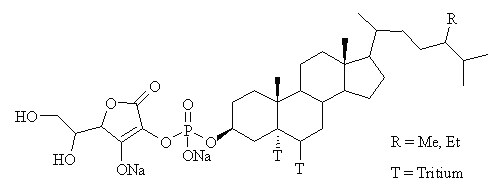
Plant sterols (known as phytosterols) and their analogues have been shown to reduce plasma cholesterol by decreasing the absorption of cholesterol from the gut (1-3) and thus may potentially reduce the risk of developing atherosclerosis and coronary heart disease. Recently our laboratory has synthesized a novel hydrophilic phytosterol analogue, FM-VP4, which is a water-soluble chemical entity that is derived from sterols through specific chemical modifications (Figure 1).
Figure 1: Chemical Structure of FM-VP4, composed of sitostanol- and campestanol-ascorbyl-phosphate. For FM-VP4 accumulation studies, FM-VP4 was tritiated at two sites, as shown.
FM-VP4 has been shown to reduce cholesterol absorption and turnover in the bloodstream of rats (3). Further studies have demonstrated that FM-VP4 has15 times 15-fold greater cholesterol inhibition activity than free sterols (2,3).
Numerous studies have been published (4-30) in which various other phytosterol mixtures were used to reduce plasma cholesterol levels without modifying plasma HDL-cholesterol nor triglyceride levels. Our laboratory has reported similar findings following a 30-day and 60-day treatment with FM-VP4 in gerbils (2,31). In addition, in these studies Additionally, low-density lipoprotein (LDL)-cholesterol levels were decreased to a relatively greater extent than total plasma cholesterol levels without evidence of renal and liver toxicity (31). In another study our laboratory observed that when rats were co-administered FM-VP4 with radiolabeled and cold unlabeled cholesterol incorporated into a lipid emulsion (Intralipid), the area under [ 3 H]cholesterol concentration versus time curve and maximum plasma concentration of [ 3 H]cholesterol were decreased in a dose-dependent manner (3). Taken together these studies suggest that FM-VP4 is a potent inhibitor of cholesterol absorption and transport into the blood. However, the mechanism(s) by which this compound decreases cholesterol gastrointestinal absorption, resulting in lower total plasma and LDL cholesterol levels, remains unknown.
One possible explanation for these findings may be a result of inhibition and/or displacement by FM-VP4 of cholesterol from cholesterol-containing micelles formed with bile acids in the gut, by FM-VP4 resulting in less cholesterol being available for absorption. To test this hypothesis, the effect of FM-VP4 on pancreatic lipase activity, an enzyme that breaks down triglycerides into fatty acids required to form cholesterol micelles, was determined. In addition, using transmission electron microscopy (TEM), cholesterol micelle formation and integrity were determined in the presence and/or absence of absence and presence FM-VP4.
Alternatively, FM-VP4 may competitively inhibit the accumulation of cholesterol from micelles into intestinal epithelial cells once contained within micelles it is possible that cholesterol's subsequent uptake into intestinal epithelial cells might well be competitively inhibited by FM-VP4. To test this hypothesis, cholesterol micelles containing unlabeled cholesterol as well as trace amounts of [ 3H]cholesterol were incubated with human colon carcinoma cell line (Caco-2 cells) cells 3cholesterol in the absence or presence or absence of increasing concentrations of FM-VP4. and c[ 3H]Cholesterol accumulation into the cell monolayers was determined. In addition, [ 3H]FM-VP4 accumulation within Caco-2 cell monolayers was determined in the presence of unlabeled cholesterol. Caco-2 cells were chosen for these studies because they represent a well-established model system for the investigation of transport across the small intestinal epithelium as reported by other investigators (32,33).
Materials and methods
Materials
The human Caco-2 cell line was obtained from the American Type Culture Collection (Manassas, VA). Tissue culture media and reagents were obtained from Sigma-Aldrich Ltd. (Oakville, Ontario, Canada) and Life Technologies Inc. (Burlington, Ontario, Canada). Tissue culture flasks and transwell inserts were obtained from Corning (Cambridge, MA). [ 3H]Cholesterol (1 mCi/mL; 45 Ci/mmol) was purchased from NEN-Dupont (Wilmington, DE). Forbes Medi-Tech Inc. (Vancouver, BC, Canada) provided unlabeled FM-VP4 (composed of sitostanol:campestanol 67:33 wt/wt), which was tritium labeled by Chem Syn Laboratories (St. Louis MO, 0.946 mCi/mL; 689 mCi/mmol) (Figure 1). FM-VP4 has an aqueous solubility of 5 mg/ml and the critical micelle concentration is approximately 10 mg/ml (data not shown).
Cell Culture
Caco-2 cells were cultured in Dulbecco's minimal essential medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 292 mg/mL glutamine, 0.1mM non-essential amino acids, 100 U/mL penicillin and 100 mg/mL streptomycin at 37°C in humidified air containing 5% CO2 . The cells were seeded at a density of 6.0 x 104 /well on 12 mm non-collagen-coated polycarbonate Transwell inserts with 3.0 mm pores. The inserts were placed into twelve-well culture plates, permitting separate access to the upper and lower compartments of the monolayers. Cells were used for experiments 28 to 30 days post-seeding, by which time cells had formed confluent monolayers and expressed the characteristics of mature, differentiated enterocytes including tight junctions (34,35).
Determination of Cell Monolayer Integrity by Electrical Resistance and Lucifer Yellow Transport
Caco-2 cell monolayer integrity was measured using the MillicellR Electrical Resistance System equipped with STX-2 electrodes (Millipore Corp., Bedford, MA). Prior to use, electrodes were equilibrated in Hank's balanced salt solution (HBSS) containing 10mM N-[2-hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid (HEPES) for 15 min to minimize voltage drift. Monolayers were washed three times and equilibrated with the same solution for 30 min prior to measuring electrical resistance, expressed as ohms/cm2 .
To confirm monolayer integrity, the paracellular transfer of a known water-soluble marker, lucifer yellow, was determined. Lucifer yellow (50 mg/mL) in HBSS containing 10mM HEPES was incubated on the apical side of the monolayers. The percentage of paracellular transfer through the monolayer was determined by measuring the fluorescence (excitation 450 nm, emission 530 nm) recovered on the basolateral side of the monolayers using a Cytofluor Series 4000 (PerSeptive Biosystems, Inc., Framingham, MA).
Micelle Preparation and Evaluation
To prepare and evaluate the effects of FM-VP4 on [3H]cholesterol accumulation from micelles, 382.9 g unlabeled cholesterol and 1 mCi (48.5 ng) [ 3H]cholesterol were dissolved in 1 mL of ethanol and mixed with 1 mL of bile salt stock solution, containing 37.2 mM taurocholic and 2.7 mM oleic acids. This solution was evaporated to dryness under a nitrogen stream and reconstituted with 7.5 mL of micelle buffer solution containing 136.9 mM sodium chloride, 2.68 mM potassium chloride, 1.47 mM potassium dihydrogen phosphate and 3.21 mM disodium hydrogen phosphate. This solution was sonicated for 30 min and incubated for 5 h at 37°C. Cholesterol and FM-VP4 micelle formation was confirmed using transmission electron microscopy (TEM) (data not shown).
Measurement of Pancreatic Lipase Activity
Pancreatic lipase activity was measured using a radioactive substrate, [ 3H]triolein (Amersham Pharmacia Biotech, Baie d'Urfe, PQ, Canada) as previously described (36). The enzymatic reaction was carried out in a final volume of 50 mL assay buffer (30 mM tris-HCl, pH 8.0, 1.0 mM CaCl2 , 4 mM taurodeoxycholate) containing 62.5 ng porcine pancreatic lipase, 250 ng porcine colipase, 0.312 mM unlabeled triolein and 500 nM [3H]triolein (25 Ci per mmole) at 25°C for 10 min in the absence or presence of increasing concentrations of FM-VP4. The reaction was stopped by addition of 750 mL chloroform/methanol/heptane (12.5:14:10) followed by 250 mL of 50 mM sodium carbonate (pH 10.5). After centrifugation at 3,000 g for 10 min, 100 mL of the top phase was carefully removed and the radioactivity determined as described above. FM-VP4 was pre-incubated with porcine pancreatic lipase in the assay buffer for 1 h before assaying the enzyme activity. 1 mM Xenical (Roche Pharmaceuticals Inc. Palo Alto, CA), a known pancreatic lipase inhibitor, was also pre-incubated with the lipase as a positive control. The results are expressed as mmoles fatty acid released per min.
Measurement of cholesterol and FM-VP4 accumulation
To determine cellular accumulation of cholesterol and FM-VP4, [ 3H]cholesterol- containing micelles (50 mM cholesterol containing 1.27x10 4% [3H]cholesterol) or [3H]FM-VP4 (50 mM) was incubated on the apical side of differentiated Caco-2 cell monolayers for 1 to 4 h at 37°C in the absence or presence of increasing concentrations (10-200 mM) of unlabeled FM-VP4 or cholesterol, respectively. Following incubation, the basolateral solution was measured for Lucifer Yellow fluorescence as described above, then the apical and basolateral solutions were transferred to appropriately labeled scintillation vials. Monolayers were washed three times with cold phosphate-buffered saline (PBS), pH 7.4, with the second wash containing 0.1% bovine serum albumin (BSA). Washings were transferred to the "apical" scintillation vial then vials were counted for tritium activity as described above. Cells were lysed by incubating with 500 mL of HBSS containing 1% Triton-X for 20 min at room temperature. The insert filter was washed with 500 mL HBSS giving a total volume of 1 mL. Lysate and washings were transferred to appropriately labeled scintillation vials and counted for tritium activity.
To determine if FM-VP4 may intracellularly inhibit cholesterol cellular accumulation, FM-VP4 was pre-incubated on the apical side of Caco-2 cells for 0.25, 0.5, 1, 2 and 18 h prior to incubating cells with cholesterol-containing micelles as described above. One to four h following incubation with [3H]cholesterol-containing micelles, the percentage of cholesterol associated with the cell monolayers was determined by radioactivity as described above. Using TERR and lucifer yellow measurements we observed that taurocholic and oleic acid found in the bile acid stock solution did not induce any cellular monolayer alterations (data not shown).
Measurement of P-gp activity
The effect of FM-VP4 on p-glycoprotein (P-gp)-mediated cellular efflux was measured by incubating FM-VP4 (50 mM) with a known substrate of p-glycoprotein (rhodamine 123, 5 mM) on the basolateral side of Caco-2 cells. The percentage of rhodamine 123 that was retained within the cells monolayer and that was transferred to the apical side was determined using fluorescent detection as previous described (37). As a positive control, verapamil (50 mM), a known modulator of P-gp (37), was co-incubated with rhodamine and the percent transfer of rhodamine 123 from basolateral to apical side was determined by fluorescence. Similar studies were carried out using the specific inhibitor of P-gp, nicardipine.
Statistical Analysis
All data were statistically compared by a one-way analysis of variance (ANOVA) using Instat2 (Graph Pad, CA) except for time-course studies for cholesterol and FM-VP4 accumulation (Figure 4) where a paired t-test was performed. The Tukey-Kramer post-hoc test was used to determine significant differences among all appropriate pairs of treatments except for pancreatic lipase activity, where the Newman-Keuls test was used. Differences were considered significant if the p-value was less than 0.05.
Results and Discussion
Effect of FM-VP4 on Cholesterol Accumulation from Micelles and Pancreatic Lipase Activity
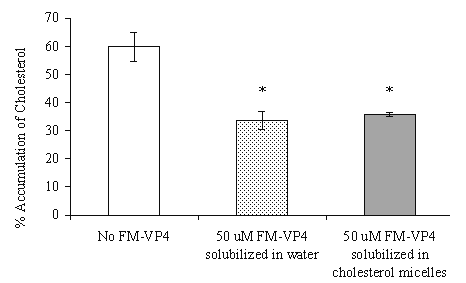
The extent of inhibition of [3H]cholesterol accumulation from micelles was not affected by the mode of FM-VP4 exposure to cells (Figure 2).
Figure 2: The effects of the delivery mode of FM-VP4 (solubilized in water or incorporated into cholesterol-containing micelles) on the accumulation of [3H]cholesterol from micelles in differentiated Caco-2 cells. *Percent accumulation of [3H]cholesterol is significantly lower than control (no FM-VP4, p<0.05), n=3 in each group.
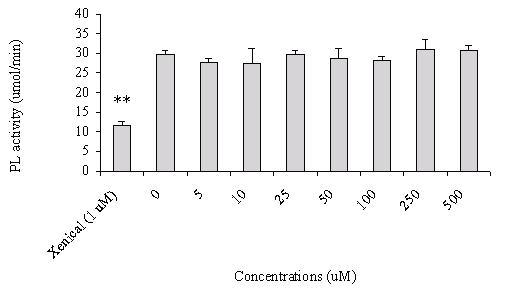
In addition, increasing concentrations of FM-VP4 (5-500 mM) did not alter pancreatic lipase activity compared to the non-treated group (Figure 3). Xenical, a known pancreatic lipase inhibitor, was used as a positive control.
Figure 3: The effect of FM-VP4 and Xenical on pancreatic lipase activity. The porcine pancreatic lipase activity was measured as described under "Materials and Methods". Different concentrations of FM-VP4 (5-500 mM) as well as 1 mM Xenical were pre-incubated with pancreatic lipase for 1 h before assaying the enzymatic activities. The results are expressed as moles fatty acid released per minute. n=6 ± S.D., **p<0.0001 compared with all other columns by Newman-Keuls Multiple Comparison test after ANOVA
Cholesterol and FM-VP4 Accumulation Studies
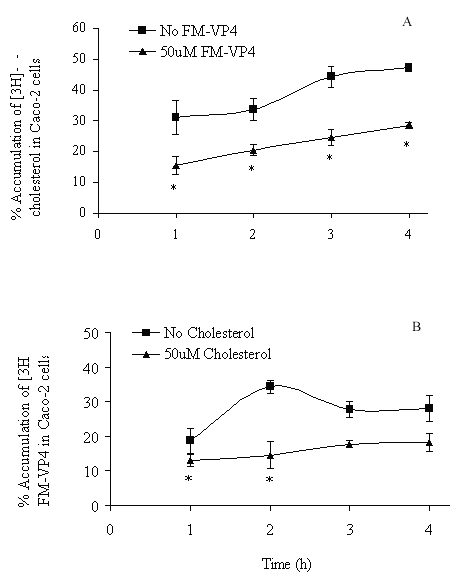
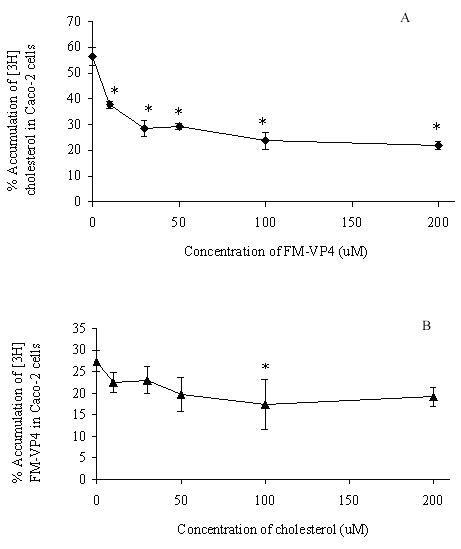
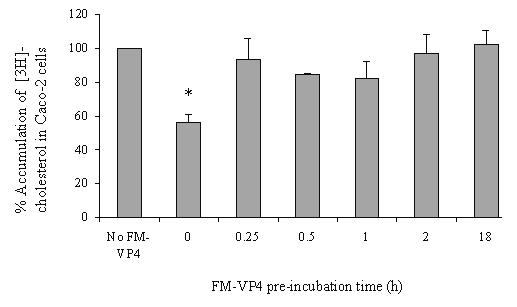
FM-VP4 at concentrations greater than 10 mM significantly inhibited cholesterol accumulation in monolayers for all incubation times tested (Figures 4A & 5A). Consistent with previous studies (38), minimal [3H]cholesterol was transported from the apical to basolateral compartment regardless of the presence of FM-VP4 (data not shown). Total [3H]cholesterol recovery was greater than 92%. Cholesterol at concentrations greater than 30 mM significantly inhibited FM-VP4 accumulation in monolayers for all incubation times tested (Figures 4B & 5B). Pre-incubation of FM-VP4 did not significantly decrease cholesterol accumulation in Caco-2 cells compared to controls (Figure 6). In contrast, a significant decrease in cholesterol accumulation (p<0.05) was observed when FM-VP4 was not pre-incubated (Figure 6). Minimal accumulation of radiolabeled cholesterol was observed on the basolateral side for all treatment groups (data not shown) as previously reported by others (30).
Effect of FM-VP4 on P-Glycoprotein Activity
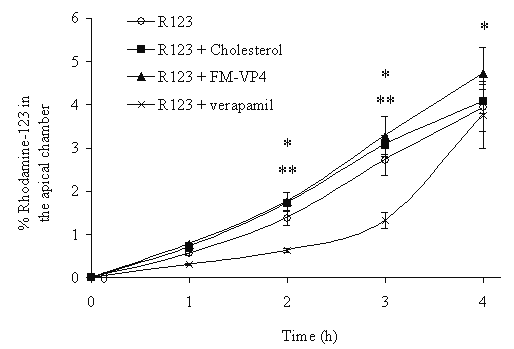
Incubating FM-VP4 (50 mM) with a known substrate of p-glycoprotein, rhodamine 123, on the basolateral side of Caco-2 cells did not alter the transport of rhodamine to the apical side. However, co-incubation of verapamil (50 mM), a known modulator of P-gp-mediated efflux of rhodamine 123, significantly decreased the transport of rhodamine from the basolateral to the apical side (Figure 7). Similar findings were observed when nicardipine was used (data not shown).
Figure 4: The effects of incubation time on A) [3H]cholesterol and B) [3H]FM-VP4 accumulation in the presence of unlabeled FM-VP4 or cholesterol, respectively. [3H]Cholesterol-containing micelles (50 mM total cholesterol with 1.27x10-4% [3H]cholesterol) or [3H]FM-VP4 (50 mM) was incubated on the apical side of differentiated Caco-2 cell monolayers for 1 to 4 h at 370C in the absence or presence of 50 mM unlabeled FM-VP4 or cholesterol, respectively. Percent accumulation is the amount of radiolabel associated with Caco-2 cells compared to the untreated control, n=3 in each group. *p<0.05 compared to control A) no FM-VP4 or B) no cholesterol for the same incubation time.
Discussion
The purpose of this investigation was to determine if a novel hydrophilic phytostanol analogue, FM-VP4, decreased the accumulation of cholesterol-containing micelles within Caco-2 cells and if so by which mechanism(s). Increasing concentrations of FM-VP4 decreased the accumulation of cholesterol-containing micelles within Caco-2 cell monolayers. In addition, decreased cholesterol accumulation occurred only when cells had not been previously exposed to FM-VP4. Furthermore, the decrease in cellular accumulation does not appear to be a result of FM-VP4 modifying the formation of cholesterol-containing micelles, pancreatic lipase activity or P-gp-mediated influx of cholesterol. However, these findings do suggest that FM-VP4 may modify some intracellular mechanisms of cholesterol influx or efflux.
Figure 5: The effects of concentration of unlabeled A) FM-VP4 and B) cholesterol on the accumulation of [3H]cholesterol and [3H]FM-VP4, respectively, in confluent Caco-2 cell monolayers. [3H]Cholesterol-containing micelles (50 μM total cholesterol with 1.27x10-4% [3H]cholesterol) or [3H]FM-VP4 (50 mM) was incubated on the apical side of differentiated Caco-2 cell monolayers for 4 h at 370C in the absence or presence of 10-200 mM unlabeled FM-VP4 or cholesterol, respectively. Percent accumulation is the amount of radiolabel associated with Caco-2 cells compared to the respective untreated control, n=3 in each group. * p<0.05 in treated groups compared to control without A) FM-VP4 or B) cholesterol
Identifying the possible mechanism(s) by which phytosterol and phytosterol analogues prevent the absorption of cholesterol from the gastrointestinal tract remains unknown. Young and Hui have recently reported that partial hydrolysis of the triacylglycerol into monoacyglycerol and non-esterified fatty acids by pancreatic lipase/colipase is required for dietary cholesterol to be absorbed as emulsified substrates by intestinal cells (39). Thus the inhibition of triacylglycerol digestion may impact on the absorption efficiency of dietary cholesterol in the gut. Therefore, we first investigated if FM-VP4 would inhibit pancreatic lipase/-mediated triacylglycerol metabolism and thus prevent dietary cholesterol absorption. We observed no inhibition of pancreatic lipase activity in the presence of FM-VP4 (Figure 3).
Figure 6: The effects of 50mM FM-VP4 pre-incubation (0, 0.25, 0.5, 1, 2 and 18 h) on [3H]cholesterol accumulation in confluent Caco-2 cell monolayers. [3H]Cholesterol-containing micelles (50 mM total cholesterol with 1.27x10-4 % [3H]cholesterol) were incubated on the apical side of differentiated Caco-2 cell monolayers after pre-incubation with FM-VP4 for up to 18 h in complete media at 370C in the absence of 50 mM cholesterol. Percent accumulation is the amount of radiolabel associated with Caco-2 cells compared to the untreated control, n=3 ineach group. *Percent accumulation of radiolabel in treated groups is significantly lower than control (no FM-VP4, p<0.05).
Some of the lipolytic products of the major pancreatic enzymes, including cholesterol, are only minimally soluble in aqueous systems and are dependent on the solubilizing properties of bile salts (40-45). When phospholipids or monoacylglycerides are present in addition to bile salts, cholesterol is solubilized into a mixed micelle solution and can readily be absorbed (40-45). Since FM-VP4 has similar structural characteristics to cholesterol (Figure 1) it was hypothesized that the presence of FM-VP4 may prevent micelle formation or cholesterol incorporation into micelles. This would reduce the extent of solubilized cholesterol thereby inhibiting cholesterol absorption in the intestinal lumen. Therefore, we investigated whether the presence of FM-VP4 during micelle formation affected the accumulation of [3H]cholesterol in differentiated Caco-2 cells. No differences were observed in the extent of cholesterol accumulation inhibition when FM-VP4 was co-incorporated within cholesterol-containing micelles or co-administered separately in water (Figure 2). This suggests that FM-VP4 does not prevent cholesterol GI absorption by inhibiting cholesterol micelle formation.
Figure 7: P-glycoprotein activity in the presence of cholesterol, FM-VP4 or verapamil, a known p-glycoprotein inhibitor, (each 50 mM) in Caco-2 cell monolayers. P-gp activity was measured by adding rhodamine 123 (5 mM) to the basolateral side and determining apical accumulation by fluorescence. Significant differences were determined by a Tukey-Kramer post-hoc test after a one-way ANOVA, n=3 in each group. *p<0.05 vs. Rhodamine 123 (R123) for all treatment groups (cholesterol, FM-VP4, verapamil) compared to control at 1 h. **p<0.05 vs. Rhodamine 123 (R123) for verapamil only compared to control at 2 and 3 h.
Concentration- and time-dependence of the inhibition of [3H]cholesterol accumulation by FM-VP4 was also determined. Increasing concentrations of FM-VP4 were found to decrease cholesterol cellular accumulation while increasing concentrations of cholesterol micelles decreased FM-VP4 cellular accumulation (Figures 4 & 5), although to a lesser extent. This suggests that FM-VP4 and cholesterol may be competing for the same site(s) on Caco-2 cells. Recently, Tessner and Stenson suggested that the overexpression of multiple drug resistance protein (MDR1) in an intestinal cell line resulted in increased cholesterol uptake (38). These findings suggested for the first time that MDR1 could function in the net influx, rather than efflux, of a substrate such as cholesterol. Due to the similarities in chemical structure it is reasonable to hypothesize that FM-VP4 and cholesterol competitively bind to MDR1 resulting in a synergistic decrease in accumulation. Alternatively, Hernandez and coworkers have recently reported that a saturable, inhibitable transporter mediates the intestinal absorption of cholesterol (46). FM-VP4 may act through this transporter to inhibit cholesterol accumulation. Additional studies to confirm these hypotheses are currently being investigated in our laboratory.
Another possible mechanism by which FM-VP4 decreases cholesterol accumulation may occur within the cell. To explore this hypothesis two strategies were used. In the first strategy, FM-VP4 was pre-incubated for different periods of time to allow for a sufficient intracellular concentration of FM-VP4 prior to incubation of cholesterol. We observed that FM-VP4 pre-incubation resulted in cholesterol accumulation levels indistinguishable from those in the absence of FM-VP4. Significant decreases in cholesterol absorption (p<0.05) occurred only when cells had not been previously exposed to FM-VP4. It is possible that FM-VP4 has an effective but short-lived intracellular effect on cholesterol efflux. Recently, a critical role for the members of the ATP-binding cassette transporter family (i.e. ABC1A, ABCG8, ABCG5) in the regulation of cholesterol absorption has been suggested (47-49). ABC1A is a member of a large family of transmembrane proteins (49-51), and it has been established to play an important role in the formation of nascent high-density lipoproteins (HDL), the initial step in reverse cholesterol transport, and in transporting cellular cholesterol and phospholipid to acceptor particles (52-55). RXR and LXR agonists have been shown to effectively increase expression of ABC1A in small intestinal mucous and to significantly inhibit cholesterol absorption (47). In addition, ABC1A knockout mice has also been shown to have an increase in cholesterol absorption (48), supporting the hypothesis that the up-regulation of ABC1A results in the inhibition of cholesterol absorption by exporting free cholesterol from inside the enterocyte into the intestinal lumen. Most recently, Berge and coworkers have determined that an increased accumulation of dietary cholesterol in an autosomal recessive disorder sitosterolemia was caused by mutations in adjacent ABC transporters, ABCG8 and ABCG5 proteins (56). FM-VP4 may inhibit cholesterol absorption through increasing expression of these ABC transporters. We hypothesize that the up-regulation of ABC transporter expression in enterocytes may contribute one of the mechanisms of action by which FM-VP4 prevent cholesterol absorption from the gut. Future studies will test this hypothesis.
Several research groups have reported that multidrug resistance P-glycoproteins may be involved in the biosynthesis and esterification of cholesterol through the transport of cholesterol from the plasma membrane to the endoplasmic reticulum (57,58). Therefore the second strategy was to determine if FM-VP4 modified cholesterol intracellular transport through p-glycoprotein. Figure 7 present findings that suggest FM-VP4 is not an inhibitor of P-gp and thus probably does not regulate intracellular cholesterol transport via this mechanism. However additional studies to investigate FM-VP4's intracellular regulation of cholesterol are warranted.
In conclusion increasing concentrations of FM-VP4 decreased the accumulation of cholesterol-containing micelles within Caco-2 cell monolayers. This decrease in was not a result of FM-VP4 modifying cholesterol micelle formation, pancreatic lipase activity or P-gp-mediated flux of cholesterol. In addition, the effectiveness of FM-VP4 was reduced when Caco-2 cells were exposed to FM-VP4 prior to incubation with cholesterol. Our findings suggest that FM-VP4 may modify some intracellular mechanisms of cholesterol influx or efflux. Additional studies to elucidate these mechanisms are currently under investigation.
Acknowledgements
Funding for this project is provided by the Canadian Institute of Health Research/Forbes Medi-Tech Inc. University/Industry Research Grant (UOP #48090 to KMW & PHP)
References
Wasan, K. M., L. Holtorf, R. Subramanian, S. M. Cassidy, P. H. Pritchard, D. J. Stewart, E. Novak, and M. Moghadasian. 2001. Assessing plasma pharmacokinetics of cholesterol following oral co-administration with a novel vegetable stanol mixture to fasting rats. J. Pharm. Sci. 90: 23-28.
Wasan, K. M., S Najafi, K. D. Peteherych, and P. H. Pritchard. 2001. Effects of a novel hydrophilic phytostanol analog on plasma lipid concentrations in gerbils. J. Pharm. Sci. In press.
Wasan, K. M., K. D. Peteherych, S. Najafi, C. Zamfir, and P. H. Pritchard. 2001. Assessing the plasma pharmacokinetics, tissue distribution, excretion and effects on cholesterol pharmacokinetics of a novel hydrophilic compound, FM-VP4, following administration to rats. J. Pharm. Pharmaceut. Sci. 4: 216-225.
Moghadasian, M. H., and J.J. Frohlich. 1999. Effects of dietary phytosterols on cholesterol metabolism and atherosclerosis: Clinical and experimental evidence. Am. J. Med. 107: 588-594.
Moghadasian, M. H., B.M. McManus, D.V. Godin, B. Rodrigues and J.J. Frohlich. 1999. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apoE-deficient mice: Possible mechanisms of action. Circulation 99: 1733-1799.
Moghadasian, M. H., B.M. McManus, P.H. Pritchard and J.J. Frohlich. 1997. "Tall-Oil"-derived phytosterols reduce atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 17: 119-126.
Heinemann, T., G. Axtmann and K. von Bergmann. 1993. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur. J. Clin. Invest. 23: 827-831.
Ikeda, I., K. Tanaka, M. Sugano, G.V. Vahouny and L.L. Gallo. 1988. Inhibition of cholesterol absorption in rats by plant sterols. J. Lipid Res. 29: 1573-1582.
Becker, M., D. Staab and K. Von Bergmann. 1993. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J. Pediatr. 122: 292-296.
Becker, M., D. Staab and K. von Bergmann. 1992. Long-term treatment of severe familial hypercholesterolemia in children: effectsof sitosterol and bezafibrate. Pediatrics 89: 138-142.
Gerson, T., F.B. Shorland and G.G. Dunckley. 1964. The effects of sitosterol on the metabolism of choleserol and lipids in rats on a diet low in fat. Biochem. J. 92: 385-390.
Gylling, H., R. Radhakrishnan and T.A. Miettinen. 1997. Reduction of serum choleserol in postmenopausal women with previous myocardial infarction and cholesterol malabsorption induced by dietary sitostanol ester margarine: women and dietary sitostanol. Circulation 96: 4266-4231.
Gylling, H., and T.A. Miettinen. 1996. Effects of inhibiting cholesterol absorption and synthesis on cholesterol and lipoprotein metabolism in hypercholesterollemic non-insulin dependent diabetic men. J. Lipid Res. 37: 1776-1785.
Gylling, H., and T.A. Miettinen. 1999. Cholesterol reduction by different plant stanol mixtures and with variable fat intake. Metabolism 48: 575-580.
Gylling, H., M.A. Siimes and T.A. Miettinen. 1995. Sitostanol ester margarine in dietary treatment of children with familial hypercholesterolemia. J. Lipid Res. 36: 1807-1812.
Gylling, H., and T.A. Miettinen. 1994. Serum choleterol and cholesterol and lipoprotein metabolism in hypercholesterolemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia. 37: 773-780.
Hallikainsen, M. A., and M.I. Uusitupa. 1999. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am. J. Clin. Nutr. 69: 403-410.
Miettinen, T. A., P. Puska, H. Gylling, H. Vanhanen and E. Vartiainen. 1995. Reduction of serum cholesterol with sitostanol-ester margarine is a mildly hypercholesterolemic population. New England J. Med. 333: 1308-1312.
Moghadasian, M. H., L.B. Nguyen, S. Shefer, B.M. McManus and J.J. Frohlich. 1999. Histologic, hematologic, and biochemical characteristics of apo E-deficient mice: effects of dietary cholesterol and phytosterols. Lab Invest. 79: 355-364.
Moghadasian, M. H., D.V. Godin, B.M. McManus and J.J. Frohlich. 1999. Lack of regression of atherosclerotic leisions in phytosterol-treated apo E-deficient mice. Life Sci. 64: 1029-1036.
Hendriks, H. F. J., J.A. Weststrate, T. van Vliet and G.W. Meijer. 1999. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolemic and mildly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 53: 319-327.
Jones, P. J. H., F.Y. Ntanios, M. Raeini-Sarjaz and C.A. Vanstone. 1999. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic man. Am. J. Clin. Nutr. 69: 1144-1150.
Konlande, J. E., and H. Fisher. 1969. Evidence for a nonabsorptive antihypercholesterolemic action of phytosterols in the chicken. J. Nutr. 98: 435-442.
Peterson, D. W. 1951. Effect of soybean sterols in the diet on plasma and liver cholesterol in chicks. Proc. Soc. Biol. Med. 78: 143-147.
Vanhanen, H. T., J. Kajander, H. Lehtovirta and T.A. Miettinen. 1994. Serum levels, absorption efficacy, fecal elimination and synthesis of cholesterol during increasing dosing of dietary sitostanol esters in hypercholesterolemic subjects. Clin. Sci. 87: 61-67.
Vanhanen, H. T., S. Blomqvist, C. Ehnholm, M. Hyvonen, M. Jauhiainen, I. Torstila and T.A. Miettinen. 1993. Serum cholesterol, cholesterol precursors, and plant sterols in hypercholesterolemic subjects with different apo E phenotypes during dietary sitostanol ester treatment. J. Lipid Res. 34: 1535-1544.
Weisweller, P., V. Heinemann, and P. Schwandt. 1984. Serum lipoproteins and lecithin:cholesterol acyltransferase (LCAT) activity in hypercholesterolemic subjects given b-sitosterol. Int. J. Clin. Pharmacol. 22: 204-206.
Aviram, M., and K. Eias. 1993. Dietary olive oil reduces low-density lipoprotein uptake by macrophages and decreases the susceptibility of the lipoprotein to undergo lipid peroxidation. Ann.Nutr. Metab. 37: 75-84.
Bhadra, S., and M.T. Subbiah. 1991. Incorporation of liposomal phytosterols into human cells in culture: a potential in vitro model for investigating pathological effects of phytosterolemia. Biochem. Med. Met. Biol. 46: 119-124.
Field, F. J., E. Born and N.M. Satya. 1997. Effects of micellar sitosterol on cholesterol metabolism in Caco-2 cells. J. Lipid Res. 38: 348-360.
Wasan, K. M., S. Najafi, J. Wong, M. Kwong, and P. H. Pritchard. 2001. Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water soluble phytostanol compound, FM-VP4, to gerbils. J. Pharm. Pharmaceut. Sci. 4: 228-234.
Gan, L.S.L., and D. K. Thakker. 1997. Applications of the Caco-2 model in the design and development of orally active drugs: Elucidation of biochemical and physical barriers posed by the intestinal epithelium. Adv. Drug Del. Rev. 23: 77-98.
Gao, J., O. Murase, R. L. Schowen, J. Aube, and R. T. Borchardt. 2001. A functional assay for quantitation of the apparent affinities of ligands of p-glycoprotein in Caco-2 cells. Pharm. Res. 18: 171-176.
Hilgers, A. R., R.A. Conradi, and P.S. Brown. 1990. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 1990 7: 902-910.
Hidalgo, I. J., K.M. Hillgren, G.M. Grass and R.T. Borchardt. 1991. Characterization of the unstrirred water layer in Caco-2 cell monolayers using a novel diffusion apparatus. Pharm. Res. 18: 222-227.
Lowe, M. E. 1999. Assays for pancreatic TG lipase and colipase. Methods Mol. Biol. 109: 59-70.
Yumoto, R, T. Murakami, Y. Nakamoto, R. Hasegawa, J. Nagai and M. Takano. 1999. Transport of rhodamine 123, a P-glycoprotein substrate across rat intestine and Caco-2 cell monolayers in the presence of cytochrome P-450 3A-related compounds. J. Pharm. Exp. Ther. 1999. 289: 149-155.
Tessner, T.G., and W.F. Stenson. 2000. Overexpression of MDR1 in an intestinal call line results in increased cholesterol uptake from micelles. Biochem. Biophys. Res. Comm. 267: 565-71.
Young, S.C., and D. Y. Hui. 1999. Pancreatic lipase/co-lipase-mediated triacylglycerol hydrolysis is required for cholesterol transport from lipid emulsions to intestinal cells. Biochem J. 339: 615-620.
Cohen, D. E., M. Angelico, M.C. Carey. 1989. Quasielastic light scattering evidence for vesicular secretion of biliary lipids. Am. J. Physiol. 257: G1-G8.
Chiba, T., S. Uematsu, F. Sawamura, M. Sugawara, I. Tomita, F. Kajiyama, T. Tomita. 1999. Effects of phosphatidylcholine/phosphatidylethanolamine composition in cholesteryl ester-micellar substrates on neutral cholesterol esterase activity. Anal. Biochem. 268: 238-244.
Luk, A. S., E.W. Kaler, and S.P. Lee. 1997. Structural mechanisms of bile salt-induced growth of small unilamellar cholesterol-lecithin vesicles. Biochem. 36: 5633-5644.
Lichtenberg, D., S. Ragimova, A. Bor, S. Almong, C. Vinkler, Y. Peled, and Z. Halpern. 1990. Stability of mixed micellar systems made by solubilizing phosphatidylcholine-cholesterol vesicles by bile salts. Hepatology 12: 149S-153S.
Cohen, B. I., T. Mikami, N. Ayyad, Y. Mikami, E.H. Mosbach. 1995. Dietary fat alters the distribution of cholesterol between vesicles and micelles in hamster bile. Lipids 30: 299-305 (1995).
Saunders, D. R., M.A. Wells. 1969. The cholesterol solubilizing capacity of lecithins in aqueous solutions of bile salt. Biochim. Biophys. Acta 176: 828-835.
Hernandez, M., J. Montenegro, M. Steiner, D. Kim, C. Sparrow, P.A. Detmers, S.D. Wright, and Y. Chao. 2000. Intestinal absorption of cholesterol is mediated by a saturable, inhibitable transporter. Biochim. Biophys. Acta. 1486: 232-242.
Repa, J. J., S.D. Turley, J.M. Lobaccaro et al. 2000. Regulation of Absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289: 1524-1529.
McNeish J, Aiello R.J., Guyot D., Turi T., Gabel C., Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. 2000. High-density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97: 4245-4250.
Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G. 1994. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics. 21: 150-159.
Klein I, Sarkadi B, Varadi A. 1999. An inventory of the human ABC proteins. Biochim. Biophys. Acta. 1461: 237-262.
Borst P, Zelcer N, van Helvoort A. 2000. ABC transporters in lipid transport. Biochim. Biophys. Acta. 1486: 128-144.
Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Hayden MR. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336-345.
Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 22: 347-351.
Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352-355.
Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 104: R25-31.
K.E. Berge, H. Tian, G.A. Graf, L. Yu, et al. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290: 1771-1775.
Metherall, J. E., H. Li, and K. Waugh. 1996. Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J. Biol. Chem. 271: 2634-2640.
Debry, P., E. A., Nash, D. W. Neklason, and J. E. Metherall. 1997. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J. Biol. Chem. 272: 1026-1031.
Corresponding Author: Kishor M. Wasan, Associate Professor & Chair, Division of Pharmaceutics and Biopharmaceutics, Faculty of Pharmaceutical Sciences, University of British Columbia, 2146 East Mall Avenue, Vancouver, British Columbia, Canada V6T 1Z3. kwasan@interchange.ubc.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps