J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(3):235-243, 2001
Guar gum as a carrier for colon specific delivery; influence of metronidazole and tinidazole on in vitro release of albendazole from guar gum matrix tablets
Krishnaiah YSR1, Seetha Devi A, Nageswara Rao L, Bhaskar Reddy PR, Karthikeyan RS, Satyanarayana V
Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam, IndiaReceived July 18, 2001, Revised August 29, 2001, Accepted August 29, 2001
PDF Version
Abstract
Purpose: The present investigation is to study the influence of metronidazole and tinidazole on the usefulness of guar gum, a colon-specific drug carrier based on the metabolic activity of colonic bacteria, using matrix tablets of albendazole (containing 20% of guar gum) as a model formulation.
Methods: The matrix tablets of albendazole were subjected to in vitro drug release studies in simulated colonic fluids (4%w/v of rat caecal contents) obtained after oral treatment of rats for 7 days either with varying doses of metronidazole/ tinidazole and 1 mL of 2%w/v of guar gum or with 1 mL of 2%w/v of guar gum alone (control study) after completing the dissolution study in 0.1 M HCl (2 h) and pH 7.4 Sorensen's phosphate buffer (3 h).
Results: The guar gum matrix tablets of albendazole were found degraded by colonic bacteria of rat caecal contents and released about 44% of albendazole in simulated colonic fluids (control study) at the end of 24 h indicating the susceptibility of the guar gum formulations to the rat caecal contents. However, the release of albendazole decreased when the drug release studies were carried out in caecal contents of rats treated for 7 days with either metronidazole (10-50 mg/ kg once daily) or tinidazole (10-30 mg/ kg once daily), and the release of albendazole from the matrix tablets was found to be dose dependent. The release of the drug from guar gum formulations was found to increase with a decrease in the dose of metronidazole/tinidazole administered. The antimicrobial activity of metronidazole/ tinidazole against the anaerobic bacteria of the rat's GI flora might have been inhibited to a varying degree depending on the dose of metronidazole/tinidazole administered.
Conclusions: The results of the study showed that concomitant administration of either metronidazole or tinidazole with guar gum based colon-specific drug delivery systems may interfere with the targeting of drugs to colon.
Introduction
Various approaches (1-2) available for colon specific drug delivery include (i) coating with pH dependent systems (ii) design of timed release dosage forms and (iii) the use of carriers that are degraded exclusively by colonic bacteria. The pH dependent systems (3-4) are designed to release the drug to above a particular pH of the GIT. The poor site specificity of pH dependent systems, because of large variation in the pH of the GIT, was very well established. The timed-release systems (5-6) release their load after a predetermined time period of administration. The site specificity of these systems is considered poor because of large variations in gastric emptying time (7) and passage across the ileocaecal junction (8). The best alternative approach for colon specific drug delivery is the use of carriers that are degraded exclusively by colonic bacteria. The microflora of colon is in the range of 1011 -1012 CFU/mL (9) consisting mainly of anaerobic bacteria, e.g. Bacteroides, Bifidobacteria, Eubacteria, Clostridia, Enterococci, Enterobacteria and Ruminococcus etc. This vast microflora fulfills its energy needs by fermenting various types of substrates that have been left undigested in the small intestine, e.g. di- and tri-saccharides, polysaccharides etc. (10-11). For this fermentation the microflora produces a vast number of enzymes like b-glucoronidase, b-xylosidase, a-arabinosidase, b-galactosidase, nitroreductase, azareducatase, deaminase, and urea dehydroxylase (12). Because of the presence of the biodegradable enzymes only in the colon, the use of biodegradable polymers for colon-specific drug delivery seems to be a more site-specific approach as compared to other approaches. These polymers shield the drug from the environments of stomach and small intestine and are able to deliver the drug to the colon. On reaching the colon, they undergo assimilation by micro-organism (13) or degradation by enzyme (14-15) or break down of the polymer back bone (16-17) leading to a subsequent reduction in their molecular weight and thereby loss of mechanical strength. They are then unable to hold the drug entity any longer (18).
The present investigation is aimed at using the inexpensive, naturally and abundantly available guar gum for colon targeted drug delivery. Guar gum is a natural nonionic polysaccharide derived from seeds of Cyamopsis tetragonolobus (Family: Leguminaciae). It consists of linear chains of (1®4)-b-D mannopyranosyl units with a-D-galactopyranosyl units attached by (1®6) linkages (19). Guar gum is used as a binder (up to 10%) and disintegrating agent in solid dosage forms. It is also used as a suspending, thickening and stabilising agent (up to 2.5%) in liquid oral and topical products.
Guar gum contains about 80% galactomannan, 12% water, 5% protein, 2% acid insoluble ash, 0.7% ash and 0.7% fat. Guar gum hydrates and swells in cold water forming viscous colloidal dispersion or sols (20-22). This gelling retards the drug release from the tablets (23-25). Guar gum is being used to deliver drug to colon due to its drug release retarding property and susceptibility to microbial degradation in the large intestine (26-28). The anaerobic bacteria that are responsible for the degradation of guar gum in the colon are Bacteroides species (B. fragilis, B. ovatus, B. Variabilis, B. uniformis, B. distasonis and B. thetaiotaomicron) .
Metronidazole and tinidazole are the drugs of choice in the treatment of amoebiasis, and are also effective against the anaerobic microorganisms (29). The concomitant use of metronidazole or tinidazole with guar gum based colon targeted formulations (e.g. guar gum matrix tablets of 5-ASA) may not be uncommon. It is to be noted that the utility of guar gum as a colon-specific drug delivery carrier is based on its degradation by colonic bacteria (30-32). The colon is rich in anaerobic bacteria (33). It implies that guar gum in the form of either a matrix tablet or as a compression coat over the drug core might have been degraded to a larger extent by the action of anaerobic microbial population of large intestine (30-32). Since metronidazole and tinidazole are active against anaerobic bacteria (29), the usefulness of guar gum on concomitant administration of these drugs with guar gum based formulations in providing colon-specific drug delivery is not known. In the light of this information, it is planned to study the influence of metronidazole and tinidazole on the usefulness of guar gum as a carrier for colon-specific drug delivery using matrix tablets of albendazole containing 20% guar gum as model formulations. It was reported that matrix tablets of albendazole containing 20% of guar gum were found to be potential colon-specific drug delivery systems in the treatment of helminthiasis (34).
Experimental
Materials
Albendazole (98.6 to 101.4% purity), mebendazole (98-101% purity), metronidazole (98.1 to 99.8% purity) and tinidazole (97.3 to 99.1% purity) were gift samples from M/s. Indechemie Laboratories, India, M/s. CIPLA Ltd., Bangalore, M/s. Alkem Laboratories (India) Limited, Mumbai, India and M/s. East India Pharmaceutical Works Limited, Kolkata, India respectively. Guar gum (viscosity of 1% aqueous dispersion is 125 cps; particle size <75 mm) was obtained from Dabur Research Foundation, New Delhi, India and was of pharmacopoeia quality (USNF). Acetonitrile (HPLC grade) and glacial acetic acid (AR) were obtained from M/s. Qualigens Fine Chemicals, Mumbai, India. Triple distilled water (TD water) was used. Other materials used in the study such as microcrystalline cellulose (Avicel, FMC Type pH-105), starch, magnesium stearate and talc were of pharmacopoeia quality (USNF).
Methodology
Preparation of Albendazole Matrix Tablets
Matrix tablets of albendazole containing 20% of guar gum were prepared by wet granulation method as described previously (34). Microcrystalline cellulose (MCC) was used as diluent and a mixture of talc and magnesium stearate (2:1 ratio) was used as lubricant. The composition of the matrix formulations used in the study containing 200 mg of albendazole is shown in Table 1. Guar gum was sieved (<250 mm) separately and mixed with albendazole (<150 mm) and MCC (<250 mm). The powders were blended and granulated with 10% starch paste. The wet mass was passed through a mesh (1680 mm) and the granules were dried at 50°C for 2 hours. The dried granules were passed through a mesh (1190 mm) and these granules were lubricated with a mixture of talc and magnesium stearate (2:1). The lubricated granules were compressed at a compression force of 4500-5500 kg using 11 mm round, flat and plain punches on a single station tabletting machine (M/s Cadmach Machinery Co. Pvt. Ltd., India). Compressed matrix tablets were compressed (100 No.) and tested for their hardness, drug content and drug release characteristics with a suitable number of tablets for each test. The hardness of the matrix tablets was determined by using Monsanto Hardness Tester.
Table 1: Composition of albendazole matrix tablets containing 20% of guar gum.
Ingredients
Quantity (mg)
present in each tabletAlbendazole
200.0
Guar gum
90.0
MCC
101.5
Starch (added as paste)
45.0
Talc
9.0
Mg stearate
4.5
Total
450.0
HPLC Analysis of Albendazole In Matrix Tablets And Dissolution Fluids
The quantitative determination of albendazole was performed by High Performance Liquid Chromatography (HPLC). A gradient HPLC (Shimadzu HPLC Class VP series) with two LC-10AT VP pumps, variable wave length programmable UV/VIS Detector SPD-10A VP, CTO-10AS VP Column oven (Shimadzu), SCL-10A VP system controller (Shimadzu) and RP C-18 column (250 mm x 4.6 mm I.D.; particle size 5 mm; YMC Inc., USA) was used. The HPLC system was equipped with the software "Class-VP series version 5.03 (Shimadzu)".
The mobile phase used was a mixture of acetonitrile and triple distilled water (TD water) containing 0.4% of triethylamine (pH adjusted to 3.6 with 5% orthophosphoric acid) in the ratio of 46:54. The filtered mobile phase was pumped at a flow rate of 1.2 mL/min. The column temperature was maintained at 40°C. The eluent was detected by UV detector at 254 nm and the data were acquired, stored and analyzed with the software Class-VP series version 5.03 (Shimadzu). A standard curve was constructed for albendazole in the range of 1 to 40 mg/mL using mebendazole as internal standard. A good linear relationship was observed between the concentration of albendazole and the ratio of the peak area of albendazole to that of mebendazole (internal standard) with a high correlation coefficient (r=0.9999). The required studies were carried out to estimate the precision and accuracy of this HPLC method of analysis of albendazole. The standard curve constructed as described above was used for estimating albendazole either in the matrix tablets or in dissolution fluids.
Determination of drug content
The albendazole matrix tablets were tested for their drug content. Ten tablets were finely powdered; 100 mg of the powder was accurately weighed and transferred to 100-mL volumetric flask. Initially about 50 mL of glacial acetic acid was added to the volumetric flask and allowed to stand for 6 h with intermittent sonication to ensure complete solubility of the drug. Then the volume was made up to 100 mL with glacial acetic acid, the mixture was centrifuged, 1 mL of the supernatant liquid was suitably diluted, filtered and analysed for albendazole content by reverse phase HPLC method as described above.
Procedure for studying the influence of oral treatment of rats with tinidazole or metronidazole on guar gum tablets of albendazole
In vitro drug release studies were carried out on guar gum matrix tablets of albendazole in the presence of 0.1 M HCl (2 h), pH 7.4 Sorensen's buffer (3 h) and in pH 6.8 phosphate buffered saline containing 4%w/v of rat caecal contents obtained after 7 days of oral treatment with both 1 mL of 2% of guar gum dispersion and metronidazole or tinidazole. The drug release studies were repeated with pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats (simulated colonic fluids) orally treated for 7 days only with 1 mL of guar gum dispersion (control). The mean percent of albendazole released in the presence of rat caecal contents obtained with and without metronidazole/ tinidazole treatment was compared to assess whether the concomitant administration of these drugs could affect the usefulness of guar gum as colon-specific carrier or not.
Preparation of simulated colonic fluids-I
Male albino rats (supplied by M/s Ghosh Enterprises, Kolkata, India) weighing 105-115 g and maintained on a normal diet (Bengal gram purchased in local market and soaked in water, 25 g/rat) were used for the study. It was reported earlier from our laboratory that rat caecal content medium at 4%w/v level obtained after 7 days of enzyme induction with 1 mL of 2% w/v guar gum dispersion provide the best conditions for assessing the susceptibility of guar gum to colonic bacterial degradation [30]. Hence, the rats were treated with guar gum dispersion for inducing the enzymes specifically acting on guar gum. The procedure involved oral treatment of rats with 1 mL of 2%w/v guar dispersion for 7 days. Thirty minutes before the commencement of drug release studies, six rats were euthanized, using carbon dioxide asphyxiation. The abdomen were opened, the caecai were traced, ligated at both ends, dissected and immediately transferred into pH 6.8 PBS, previously bubbled with CO2. The caecal bags were opened, their contents were individually weighed, pooled and then suspended in PBS to give 4%w/v dilution. As the caecum is naturally anaerobic, all these operations were carried out under CO2. The care of the rats was in accordance with institutional guidelines.
Preparation of simulated colonic fluids-II
On each day the rats were administration orally with 1 mL of 2%w/v guar gum dispersion and after 12 h, they were treated with either metronidazole or tinidazole suspended in 1% NaCMC in different doses. This treatment schedules was followed for 7 days. Metronidazole was administered orally once daily at the dose levels of 10, 30 or 50 mg/kg whereas tinidazole was administered at the dose levels of 10, 15 or 30 mg/kg once daily for 7 days. On eighth day, thirty minutes before the commencement of drug release studies, six rats were euthanized, using carbon dioxide asphyxiation and the caecal contents were obtained described previously.
In vitro drug release studies
The susceptibility of guar gum matrix tablets of albendazole to the enzymatic action of colonic bacteria was assessed by continuing the drug release studies in 100 mL of simulated colonic fluids-I (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated only with guar gum dispersion) after completing the first five hours of study in both 0.1 M HCl (900 mL; 2 h) and pH 7.4 Sorensen's phosphate buffer (900 mL; 3 h). The drug release studies were carried out in a USP dissolution rate test apparatus (Apparatus 1, 100 rpm, 37°C) with slight modification. A beaker (capacity 150-mL) containing 100 mL of dissolution medium was immersed in the water contained in the 1000-mL vessel, which was, in turn, in the water bath of the apparatus. The tablets were placed in the baskets of the apparatus and immersed in the dissolution medium containing rat caecal contents. The experiment was carried out with continuous CO2 supply into the medium to simulate anaerobic environment of the caecum.
The amount of drug in the entire quantity of the dissolution medium, i,.e., either 900 mL of 0.1 M HCl, 900 mL of pH 7.4 phosphate buffer or 100 mL of rat caecal content medium (simulated colonic fluids-I or simulated colonic fluids-II) at the end of 2, 5, 10, 15 and 24 h respectively was estimated by HPLC. In case of drug estimation in 0.1 M HCl and pH 7.4 phosphate buffer, the entire quantity of dissolution medium (900 mL) after dissolution study was transferred to 1000-mL volumetric flask containing glacial acetic acid (about 70 mL). The contents were sonicated well for complete dissolution of albendazole and made upto volume with glacial acetic acid. One milliliter of this mixture was transferred to 10-mL volumetric flask containing 1 mL of mebendazole solution (20 mg), made upto volume with respective blank dissolution fluids, filtered through 0.4 mm membrane filter and the filtrate was subjected to HPLC analysis as described above.
The drug released in rat caecal content medium was estimated by HPLC as follows. The entire quantity of rat caecal content medium (100 mL) at the end of the study was transferred to 250-mL volumetric flask containing about 100 mL of glacial acetic acid. The contents were shaken to completely dissolve the eroded drug particles and made upto volume. One milliliter of the sample from this flask was added to 10-mL volumetric flask containing 1 mL of mebendazole (20 mg), made upto volume, transferred to centrifuge tubes, centrifuged at 2,500 rpm for 15 min, supernatant liquid was filtered through 0.4 mm membrane filter and the filtrate was subjected to HPLC analysis as described above.
The drug content remained in either the mass of the formulation or swollen formulation was also determined by HPLC to account for the total amount of drug present in the formulation. This ensures the estimation of all the finely suspended drug particles that may be released from the guar gum matrix formulation on erosion by colonic bacteria.
The in vitro drug release studies were repeated in the same way as described above in the presence of simulated colonic fluids-II (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated with both guar gum dispersion and metronidazole/ tinidazole). However, the drug release was measured only at 2, 5 and 24 h.
Statistical analysis
The mean percent of albendazole released in simulated colonic fluids-II (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated with both guar gum dispersion and metronidazole/ tinidazole) was compared with that of the drug released in simulated colonic fluids-I (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated with guar gum dispersion only). Students t-test was used to find the statistical significance. A value of P<0.05 was considered statistically significant.
Results and Discussion
The present study was aimed at finding the influence of metronidazole and tinidazole on the usefulness of guar gum as a carrier for colon-specific drug delivery using guar gum matrix tablets of albendazole as model formulation. It was reported earlier that matrix tablets of albendazole containing 20% of guar gum were potential in targeting albendazole to colon in the treatment of helminthiasis (34). The matrix tablets were prepared by applying maximum force of compression and the hardness of the tablets was found to be in the range of 5 to 6 kg/cm2 . When subjected to content uniformity test, the matrix tablets were found to contain 98-101% of albendazole indicating the uniformity of the drug content. Albendazole tablets were subjected to in vitro dissolution study in the presence of either simulated colonic fluids-I or simulated colonic fluids-II after completing the first 5 h of study in 0.1 M HCl (900 mL; 2 h) and pH 7.4 Sorensen's phosphate buffer (900 mL; 3 h). When the drug content remained either in the tablet mass or in the swollen matrix formulation was determined and summed up with the total drug released in the dissolution study, it was found equal to the total amount of drug (albendazole) present in the formulation. This ensured the estimation of all the drug particles that might have eroded during the in vitro dissolution study.
In the presence of caecal contents of rats treated with guar gum only (simulated colonic fluids-I), the formulation degraded into 2 to 3 pieces at end the of 24 h of study thereby releasing about 44% of albendazole. The percent of drug released from guar gum matrix tablets of albendazole in the presence of colonic simulated fluids-I is shown in Table 2. The formulation was found to release about 29% of albendazole in the dissolution medium containing caecal contents of rats treated with guar gum dispersion. But in control study it was only 9% indicating that the release is not due to mechanical erosion, but due to the anaerobic microbial enzymatic action on swollen guar gum matrix tablets (30). This is based on the reports (26-28) that the anaerobic bacteria responsible for the degradation of guar gum in the colon are Bacteroides species (B. fragilis, B. ovatus, B. Variabilis, B. uniformis, B. distasonis and B. thetaiotaomicron).
Table 2: Percent* of albendazole released (n=3) from the matrix tablets containing 20% guar gum without and with rat caecal contents
Time (h)
Percent of albendazole released
without rat caecal contents
with rat caecal contents
2
8.3± 0.7
8.4± 0.1
5
11.9± 1.6
12.4± 2.1
10
12.05± 0.6
15.3± 0.3
15
16.2± 0.3
20.1± 0.4
20
18.1± 0.1
33.1± 1.3
24
20.9± 0.6
43.9± 1.6
*: Values shown in the table indicate the mean ±s.d.
Though the present results showed only 44% of drug release from the guar gum matrix tablets of albendazole, it is possible that the formulation may release majority of the drug in the physiological environment of human colon. This assertion was based on the fact that the human caecal contents would be much higher than what was used in the present study that may release majority of the drug content in the formulation. Hence, the matrix tablets of albendazole containing 20% of guar gum were chosen as model formulation to assess the influence of metronidazole/ tinidazole treatment on the usefulness of guar gum as a carrier.
Influence of metronidazole
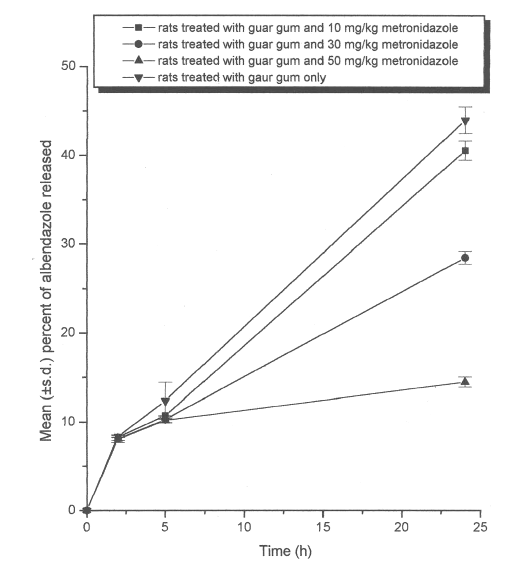
When drug release studies were conducted in simulated colonic fluids-II (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated with both guar gum dispersion and different doses of metronidazole), the microbial degradation of the guar gum matrix formulation was found to vary with the dose of metronidazole. The maximum oral dose of metronidazole administered to the rats was 50 mg/kg body weight (35). In caecal contents of rats treated with 50 mg/kg of metronidazole, the swollen guar gum matrix formulation was found intact releasing 14.5% of albendazole only (Figure 1).
Figure 1: Mean (±s.d.) percent of albendazole released from matrix tablets (n=3) containing 20% of guar gum in dissolution study without (control) and with metronidazole treatment (10, 30 or 50 mg/kg, oral once daily for 7 days).
Metronidazole treatment (50 mg/kg) significantly (P<0.001) reduced the percent of albendazole released from matrix formulation when compared to the drug released in control study. In caecal contents of rats treated with lower dose of metronidazole (30 mg/kg), the guar gum matrix formulation was found to degrade partially and released 28.5% of albendazole (Figure 1). The decrease in albendazole release was statistically significant (P<0.001) on treatment with 30 mg/kg of metronidazole. However, with a further decrease in the dose of metronidazole (10 mg/kg, oral) (Figure 1), the formulation was found to degrade into 2 to 3 pieces thereby releasing 40.5% of albendazole. There was no significant difference (P>0.05) in the percent of albendazole released after treatment with 10 mg/kg when compared to control study. Thus, the release of the drug from guar gum formulations was found to increase with a decrease in the dose of metronidazole administered (Figure 1). Due to the antimicrobial activity of metronidazole (29) against the anaerobic bacteria, the rat's GI flora might have been inhibited to a varying degree depending on the dose of metronidazole administered. The results of the study thus indicate that the concomitant administration of metronidazole with guar gum-based colon-targeted formulations is likely to interfere with the release of the drug in the colon.
Influence of tinidazole
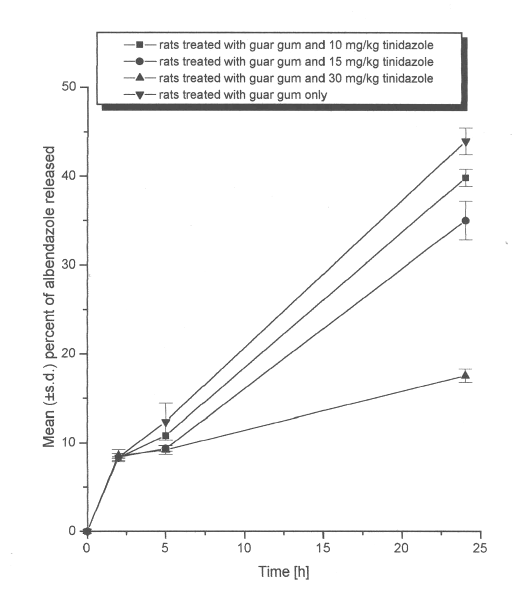
When drug release studies were conducted in simulated colonic fluids-II (pH 6.8 phosphate buffered saline containing 4%w/v of caecal contents of rats treated with both guar gum dispersion and different doses of tinidazole), the microbial degradation of the guar gum was found to vary with the dose of tinidazole.
In caecal contents of rats treated orally with 30 mg/kg of tinidazole, the swollen guar gum matrix formulation was found intact and released 17.5% of albendazole only (Figure 2). The release of albendazole decreased significantly (P<0.001) on treatment with 30mg/kg of tinidazole when compared to the drug released in control study. In caecal contents of rats treated with lower dose of tinidazole (15 mg/kg, oral), the guar gum matrix formulation was found to degrade partially releasing 34.5% of albendazole (Figure 2). There was a statistically significant (P<0.01) decrease in the amount of drug released after treatment with 15 mg/kg of tinidazole. However, with a further decreased dose of tinidazole (10 mg/kg, oral), the formulation was found to degrade into 2 pieces thereby releasing 39.8% of albendazole (Figure 2).
Figure 2: Mean (±s.d.) percent of albendazole released from matrix tablets (n=3) containing 20% of guar gum in dissolution study without (control) and with tinidazole treatment (10, 15 or 30 mg/kg, oral once daily for 7 days).
Unlike metronidazole, even with 10 mg/kg of tinidazole treatment, there was a significant (P<0.05) decrease in the percent of albendazole release from guar gum matrix formulations. Thus, the release of the drug from guar gum formulations was found to increase with a decrease in the dose of tinidazole administered (Figure 2). Due to the antimicrobial activity of tinidazole against the anaerobic bacteria (29), the rat's GI flora might have been inhibited to a varying degree depending on the dose of tinidazole administered. The results of the study thus indicate that the concomitant administration of tinidazole with guar gum based colon targeted formulations is likely to interfere with the release of the drug in the colon. In view of the results of the present study, the influence of other antimicrobial agents against anaerobic bacteria (e.g., cefoxitine, cefotitan, clindamycin) needs to be studied. Such studies are in progress.
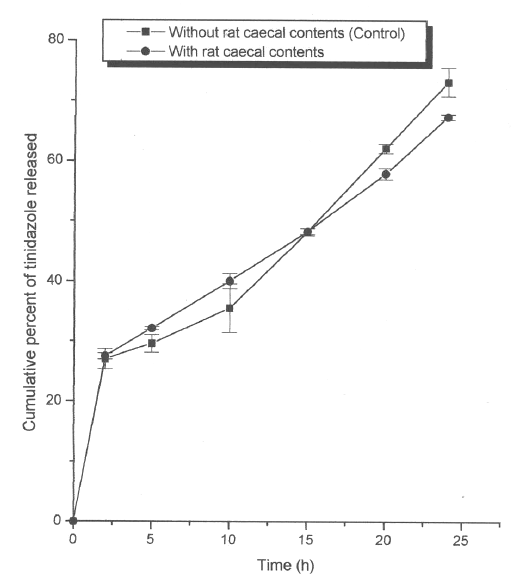
Guar gum based formulations for tinidazole are being developed in this laboratory for colon targeting in the treatment of amoebiasis. When the matrix tablets of tinidazole containing 20% of guar gum were subjected to in vitro drug release studies, there was no significant difference (Figure 3) in the percent of drug released in rat caecal contents when compared to the drug released in the absence of rat caecal contents (control).
Figure 3: Mean (±s.d.) percent of tinidazole released from matrix tablets (n=3) containing 20% of guar gum in dissolution study without (control) and with rat caecal contents.
It appeared that tinidazole released from the guar gum matrix formulation might have inhibited the anaerobic bacteria (29) present in the rat caecal contents and hence insignificant difference in the percent of drug released from the formulation. For this reason, a detailed study was undertaken to study the influence of tinidazole and metronidazole (other antiamoebic drug having antimicrobial activity against anaerobic bacteria) on the usefulness of guar gum as a carrier for colon-specific drug delivery using guar gum tablets of albendazole as model formulation.
The results of the study showed that concomitant administration of either metronidazole or tinidazole would interfere with the degradation of guar gum by colonic bacteria when these drugs are administered as guar gum matrix tablets. In the light of these results, the successful development of guar gum-based colon-targeted delivery systems containing drugs acting on anaerobic bacteria requires a tight control of the drug release from the swollen guar gum formulations in the presence of rat caecal contents. This could be achieved by compression coating of guar gum over the drug core in a quantity sufficient to provide a tight control of antiamoebic drugs until the swollen guar gum is acted upon by caecal contents of colonic bacteria. Studies are in progress in this direction, and guar gum compression coated formulations of metronidazole and tinidazole are being developed for delivering these drugs in the physiological environment of large intestine.
Conclusions
The objective of the study was to find the influence of concomitant administration of metronidazole/ tinidazole (having also antimicrobial activity against anaerobic bacteria) on the usefulness of guar gum as a carrier for colon-specific drug delivery using guar gum matrix tablets of albendazole as a model formulation. The results showed that the release of albendazole from guar gum matrix tablets decreased with an increase in the dose of metronidazole and tinidazole administered. For successful design of colon targeted delivery systems for drugs having antimicrobial activity against anaerobic bacteria using guar gum as a carrier, it requires a tight control of drug release until the swollen guar gum formulation is acted upon by colonic bacteria.
Acknowledgements
The authors acknowledge the financial support received from Government of India, Department of Science and Technology (DST), All India Council for Technical Education (under MODROBS and TAPTEC research schemes) and University Grants Commission, New Delhi. The authors greatly acknowledge M/s. Indechemie Laboratories, India, M/s. CIPLA Ltd., Bangalore, India, M/s. Alkem Laboratories (India) Limited, Mumbai, India, M/s. East India Pharmaceutical Works Limited, Kolkata, India and Dabur Research Foundation, New Delhi, India for the gift samples of albendazole, mebendazole, metronidazole, tinidazole and guar gum respectively.
References
Van den Mooter, G. and Kinget, R., Oral colon-specific drug delivery: A review. Drug Delivery, 2: 81-931, 1995.
Rama Prasad, Y.V., Krishnaiah, Y.S.R. and Satyanarayana, S., Trends in colonic drug delivery: A review. Indian Drugs, 33: 1-10, 1996.
Touitou, E. and Rubinstein, A., Targeted enteral delivery of insulin to rats. Int J Pharm, 30: 95-99,1986.
Peters, R. and Kinget, R., Film-forming polymers for colonic drug delivery I. Synthesis and physical and chemical properties of methyl derivatives of Eudragit S. Int J Pharm, 94: 125-134, 1993.
Gazzaniga, A., Sangali, M.E. and Giordano, M., Oral Chronotopic® drug delivery systems: achievement of time and/or site specificity. Eur J Pharm Biopharm, 40: 246-250, 1994.
Pozzi, F., Furlani, P., Gazzaniga, A., Davis, S.S. and Wilding, I.R., The time clock system: a new oral dosage form for fast and complete release of drug after a predetermined lag time. J Control Rel, 31: 99-108, 1994.
Davis, S.S., Hardy, J.G., Taylor, M.J., Stockwell, A., Whalley, D.R. and Wilson, C.G., The in vivo evaluation of an osmotic device (Osmet) using gamma scintigraphy. J Pharm Pharmacol, 36: 740-742, 1984.
Marvola, M., Aito, H., Ponto, P., Kanniksoki, A., Nykanen, S. and Kokkonen, P., Gastrointestinal transit and concomitant absorption of verapamil from a single unit sustained release tablet. Drug Dev Ind Pharm, 13: 1593-1609, 1987.
Moore, W.E.C. and Holdeman, L.V., Discussion of current bacteriological investigations of the relationship between intestinal flora, diet and colon cancer. Cancer Res, 35: 3418-3420, 1975.
Rubunstein, A., Microbially controlled drug delivery to the colon. Biopharm Drug Dispos, 11: 465-475, 1990.
Cummings, J.H. and Englyst, H.N., Fermentation in the human large intestine and available substrates. Am J Cli Nutr, 45: 1243-1255, 1987.
Scheline,R.R., Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol Rev, 25: 451-523, 1973.
Potts, J.E., Clendinnings, R.A., Ackard, W.B. and Wiegisch, W.D., The biodegradability of synthetic polymers. In: Guillet, J. (Ed.), Polymer science and technology, Vol. 3. Plenum Press, New York, pp. 61-79, 1973.
Huang, S.I., Bansleben, D.A. and Knox,J.R., Biodegradable polymers: Chymotrypsin degradation of low molecular weight poly (ester-urea) containing phenylalanine. J Appl Polym Sci, 23: 429-437, 1979.
Swift, G., Biodegradable polymers in the environment: are they really biodegradable? Proc ACS Div Poly Mat Sci Eng, 66: 403-404, 1992.
Ratner, B.D., Gladhill, K.W. and Horbett, T.A., Analysis of in vitro enzymatic and oxidative degradation of polyurethanes. J Biomed Mater Res, 22: 509-527, 1988.
Hergenrother, R.W., Wabewr, H.D. and Cooper,S.L., The effect of chain extenders and stbilizers on the in vivo stability of polyurethanes. J Appl Biomat, 3: 17-22, 1992.
Park, K., Shalaby, S.W.W. and Park, H., Biodegradation. In: Biodegradable hydrogels for drug delivery. Technomic publishing company, USA, pp. 13-34, 1993.
Goldstein, A.M., Alter, E.N. and Seaman, J.K., Guar gum. In: Whistler RL (Ed.). Industrial gums, polysaccharides and their derivatives. Academic Press, New York, pp 303-321, 1993.
Johnson, J.C. and Gee, J.M., Effect of gel forming gum on the intestinal unstirred layer and sugar transport in vitro. Gut, 22: 398-403, 1981.
Cheetham, N.W.H. and Mashimba, E.N.M., Conformational aspects of xanthan-galactomannan gelation. Further evidence from optical-rotation studies. Carbohydr Polym, 14: 17-27, 1991.
Brosio, E., D'ubado, A. and Verzegnassi, B., Pulsed field gradient spin-echo NMR measurement of water diffusion coefficient in thickening and gelling agents: Guar galactomannan solution and pectin gels. Cell Mol Biol, 40: 569-573, 1994.
Elsabbagh, H., Sakr, A. and Abd-Elhadi,S., Effect of guar gum on the dissolution rate of epidrine hydrochloride and sulphadimidine tablets. Pharmazie, 33: 730-731, 1978.
Bhalla, H.L. and Shah, A.A., Controlled release matrices for ketoprofen. Ind Drugs, 28: 420-422, 1991.
Jain, N.K., Kulkarni, K. and Talwar,N., Controlled release tablets formulation of isoniazid. Pharmazie, 47: 277-278, 1992.
Bayliss, C.E. and Houston, A.P., Degradation of guar gun by faecal bacteria. Appl Environ Microbiol, 48: 626-632, 1986.
Tomolin, J., Taylor, J.S. and Read, N.W., The effect of mixed faecal bacteria on a selection of viscous polysaccharide in vitro. Nutr Rep Int, 39: 121-135, 1989.
Macfarlane, G.T., Hay, S., Macfarlane, S. and Gibson, G.R., Effect of different carbohydrates on growth, polysaccharidase and glycosidase production of Bacteroides ovatus in batch and continuos culture. J Appl Bacteriol, 68: 179-187,1990.
Tracy, J.W. and Webster, T.L Jr., Drugs used in the chemotherapy of protozoal infections: in Goodman; Gilman (eds), The Pharmacological Basis of Therapeutics, 9th ed., Mc Graw Hill, New York, NY, pp. 995-998 and 1012-1015, 1996.
Rama Prasad, Y.V., Krishnaiah, Y.S.R. and Satyanarayana, S., In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Rel, 51: 281-287, 1998.
Krishnaiah, Y.S.R., Satyanarayana, S., Rama Prasad, Y.V. and Narasimha Rao, S., Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy subjects. J Control Rel, 55: 245-252, 1998.
Krishnaiah, Y.S.R., Satyanarayana. S., Rama Prasad, Y.V. and Narasimha Rao, S., Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm, 171: 137-146, 1998.
Simon, G.L. and Gorbach, S.L., Intestinal flora in health and disease. Gastroenterology, 86: 174-193, 1984.
Krishnaiah, Y.S.R., Nageswara Rao, L., Latha, K., Satyanarayana, V. and Karthikeyan, R.S., Studies on the development of guar gum matrix tablets of albendazole for colon targeting. Pharm Sci, 2001.(communicated)
Satoskar, R.S.; Bhandarkar, S.D. and Aninapur S.S Pharmacology and Pharmacotherapeutics 14th Ed, pp. 696 and 705-707, 1995.
Corresponding Author: Y.S.R. Krishnaiah, Department of Pharmaceutical Sciences, Andhra University, Visakhapatnam, India, 530 003. krishnaysr112@rediffmail.com
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps