J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(3):244-247, 2001
High Performance Liquid Chromatography of Mebudipine: Application to Pharmacokinetic Study
Shahab Bohlooli, Fariborz Keyhanfar, Massoud Mahmoudian1
Department of Pharmacology, Iran University of Medical Sciences, Tehran, IranReceived July 22, 2001, Revised September 30, 2001, Accepted October 1, 2001
PDF Version
Abstract
Purpose: To develop a high performance liquid chromatography system for the determination of a new 1,4-dihydropyridine, mebudipine, in rabbit plasma.
Methods: To 1 ml of rabbit plasma was added internal standard (dibudipine) and 0.5 ml of 1 M NaOH. Mebudipine and internal standard were extracted to 5 ml ethyl acetate, evaporated under slow stream of nitrogen. The residue was reconstituted in 200 ml mobile phase and 20 ml of aliquots were injected into a HPLC system equipped with 4.6×250 mm i.d. C18 analytical column. Mobile phase consisted of methanol (70%), water (25%) and acetonitril (5%) and its flow rate was 1 ml/min.
Results: There were no interfering peaks from endogenous components in blank plasma chromatograms. Standard curves were linear (r2>0.99) over 10 to 500 ng/ml. The extraction efficiency was >90% and the minimum quantifiable concentration was 10 ng/ml (CV<10%).
Conclusion: A suitable, convenient and simple HPLC assay for pharmacokinetic study of mebudipine in rabbits was developed.
INTRODUCTION
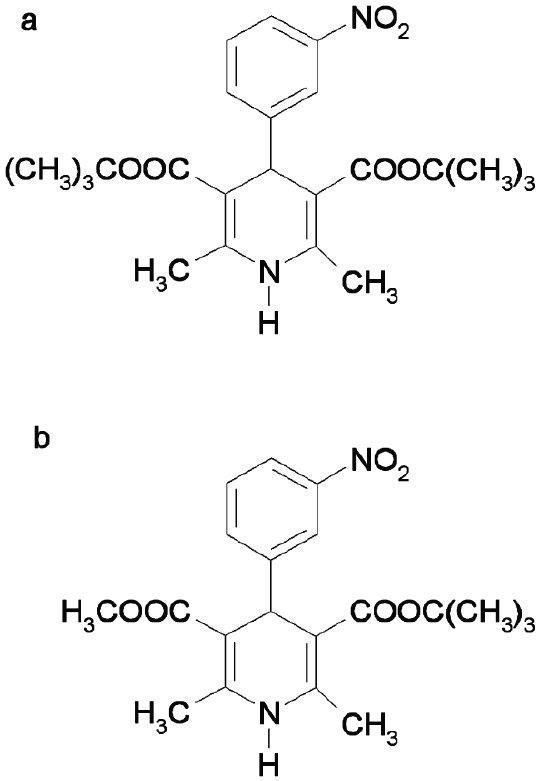
Mebudipine [(±)-t-butyl, methyl-1, 4-dihydro-2, 6-dimethyl-4 -(3-nitrophenyl)-3,5-pyridine dicarboxylate] is a new calcium channel blocker with 1, 4-dihydropyridine structure (Figure 1) that was first synthesized by Mahmoudian et al in our laboratory (1). In pervious studies it was shown that mebudipine had comparable pharmacological effect with nifedipine while offering some advantages such as longer biological half life, longer time to reach peak effect and more vasoselectivity (1, 2). This paper describes an assay method for mebudipine in plasma and its application to a preliminary pharmacokinetic study was conducted to demonstrate the usefulness of the method.
Figure 1: Chemical structure of a) dibudipine and b) mebudipine.
Materials and Methods
Chemicals
Mebudipine and dibudipine (internal standard, Figure 1) were synthesized in our laboratories as described previously (1). HPLC grade methanol, acetonitril and ethyl acetate were purchased from Merck (E. Merck, 64271 Darmstadt, Germany). All other reagents were analytical grade.
HPLC System
The HPLC system consisted of a Waters liquid chromatography system (Waters chromatography division. Milford, MA, USA) including a model 600 controller, a model 600 pump, a model 486 UV tunable absorbance detector, a model 7725i Rheodyne manual injector and a model 746 data module. An analytical column (Novapack ODS, 5 mm, 4.6 mm X 250 mm) and a guard column (C18 Waters,MA,USA) were used for all analyses.The mobile phase consist of methanol-water-acetonitril (70-25-5). The chromatography analyses were performed at ambient temperature at flow rate of 1 ml/min and the elute was monitored at 238 nm.
Sample preparation
To one ml of plasma sample were added, 10 μl of internal standard (dibudipine, 20 mg/ml) solution and 0.5 ml of 1M NaOH. The solution was mixed for a few seconds. Five ml ethyl acetate was added to the solution which was subsequently shaken on horizontal shaker for 10 min followed by centrifugation at 18000 g for 10 min.The organic layer was transferred to a clean glass tube and evaporated to dryness under nitrogen at 40°. The resultant residue was reconstituted with 200 ml of mobile phase and 20 ml was injected into the HPLC.
Extraction efficiency
Different solid phase extraction methods were examined and were found to be inappropriate due to low extraction efficiency. In addition various organic solvents were tested for the extraction procedure and ethyl acetate proved to be the most suitable because of smaller interference of endogenous components and good extraction efficiency. The extraction efficiency was calculated by adding known amount of mebudipine (10,50, 200 and 400 ng/ml, n=4) to 1 ml rabbit plasma. Mebudipine was extracted as described above, The peak heights of mebudipine from spiked plasma samples were compared with the peak heights obtained after direct injection of 20 ml of 10, 50, 200 and 400 ng/ml mebudipine solutions.
Accuracy and Precision
For the determination of intra-day and interday accuracy and precision of the assay, aliquots of 1 ml rabbit plasma were spiked with 10 ml of 20 mg/ml of internal standard and various quantities of mebudipine to yield 10,50, 200 and 400 ng/ml. Accuracy was expressed as the mean% [(mean measured concentration)/(expected concentration)]×100 (3). Precision was calculated as inter and intra-day coefficient of variation [%CV=(SD/mean)×100] (3).
Pharmacokinetic of Mebudipine in Rabbits
Three adult male albino rabbit were each administrated single bolus intravenous doses of 0.50 mg/kg mebudipine dissolved in 60% PEG 400). Blood samples were collected from marginal ear vein at 5, 10, 20, 30,60, 120, 180,240 min after mebudipine administration. Plasma was separated by immediate centrifugation and was kept at -20° until analyzed. Pharmacokinetic parameters were calculated by two-compartmental method (4) using DRUG-KNT program (5).
Results and Discussion
Acetonitril was used for denaturing of plasma proteins. Denauration with acids reduced the assay's extraction, due perhaps to ecomposition of the drug.
We tried solid phase method for extraction procedure. Initially a silica-based solid extraction cartridge was used which gave clean chromatograms. However, the method was not reproducible and the extraction efficiency was less than 50%. Consequently, a C18 extraction cartridge was employed. Mebudipine was eluted with different organic solvents from solid phase. Best result was produced when cartridge was washed with 6 ml pH 8 phosphate buffer in three 2 ml steps followed by a 3 ml acetonitril wash. Under these conditions, the assay was reproducible but the extraction efficacy was still less than 60%. Liquid phase extraction method yielded the best results. Ethyl acetate showed good extraction efficiency (>90%) and clean blank chromatograms.
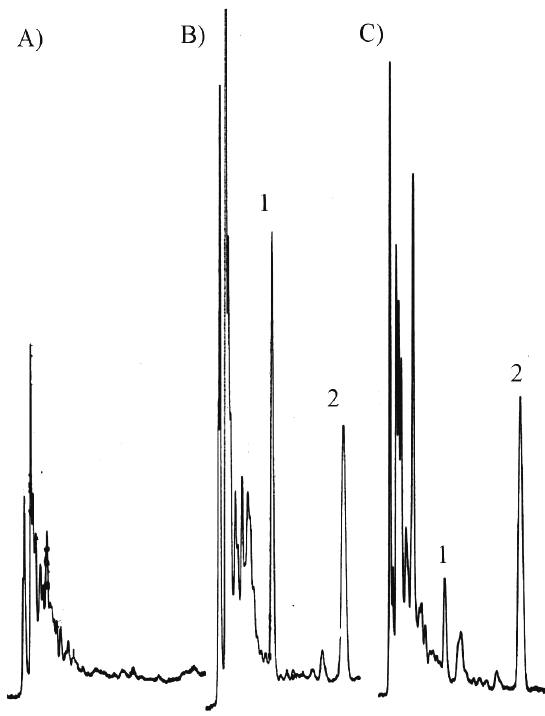
Typical chromatograms of blank plasma, spiked plasma and an actual sample obtained from the pharmacokinetic study are shown in Figure 2.
Figure 2: Chromatograms of mebudipine in rabbit plasam A.) blank rabbit plasma, B.) sample spiked with 50 ng/ml mebudipine and 200 ng/ml IS, and C.) sample obtained 20 min after an IV dose of 500 ug/ml mebudipine. Peaks: 1=mebudipine 2=dibudipine.
Retention time of mebudipine and internal standard were approximately 8 and 15 min, respectively. The total HPLC run time for each sample was about 20 min. There were no interfering peaks in the blank plasma samples. Standard curves prepared for mebudipine in rabbit plasma was linear over 10 to 500 ng/ml. The mean (n=3) calibration curve for mebudipine was y=0.008x - 0.0022, r2 =0.9989 where, y and x are the peak height ratio and concentration (ng/ml), respectively. Mebudipine concentration as low as 10 ng/ml could be quantities. The sensitivity of assay was sufficient for pharmacokinetic study. The accuracy of assay was >90% and CV did not exceed 10% (Table 1).
Table 1: Intra and interday variation of mebudipine assay in rabbit plasma.
Prepared Concentration (ng/ml)
Measured Concentration (ng/ml)
C.V. (%)
Accuracy (%)
Intraday
10
9.78
6.66
97.82
50
45.55
4.57
91.1
200
188.67
3.26
94.34
400
402.34
7.3
100.58
Interday
10
10.27
9.6
102.73
50
50.41
9.7
100.83
200
198.62
2.53
99.3
400
391.2
6.7
97.8
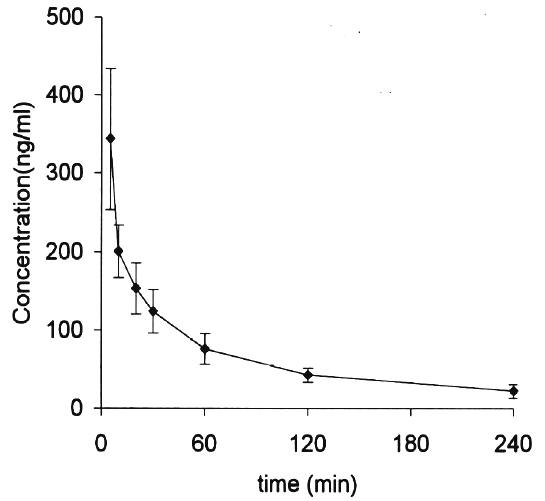
The mebudipine stock solution was stable at least for 3 months when the solution was protected from light and kept in -20° . However, when stock solutions were kept in the room temperature for approximately after two weeks, several peaks representing degradation products appeared in chromatograms. The method mentioned above was applied to pharmacokinetic study of mebudipine in rabbits. The mean plasma concentration time curve is shown in Figure 3 while corresponding pharmacokinetic parameters are reported in Table 2.
Figure 3: Mean mebudipine plasma concentration-time profile in rabbits following IV administration of 500 mg/kg mebudipine. Each point represents the mean ± SE for three rabbits.
Table 2: Pharmacokinetic parameters of mebudipine following intravenous bolus administration of 0.50 mg/kg mebudipine in three rabbits.
Animal
AUC0-4
AUC0-¥
t1/2
CLT
Vss
R1
176
214
2.03
5.31
6.8
R2
342
445
2.16
1.9
3.5
R3
177
214
2.02
5.31
5.73
Mean±SE
231±78
291±109
2.07±0.06
4.18±1.6
5.34±1.37
AUC, area under plasma concentration-time curve; CLT, total body clerance; Vss, steady-state volume of distribution
After IV administration, plasma concentration curves were bioexponential. The a and b phase declined with a mean half-life of 16 min and 134 min respectively. The total clearance of mebudipine was 4.2 l/h/kg. The volume of distribution at steady state of 5.34 l/kg indicates that mebudipine distributed into tissues to a moderate extent due, perhaps, to its lipophilic nature.
Conclusion
The HPLC assay presented here is suitable for pharmacokinetic study of mebudipine in rabbits. This assay is convenient, simple and sensitive.
ACKNOWLEGMENT
The author is thankful to Dr. Ebrahimi for technical and scientific assistance and to Dr. Motevalian for accommodation of some equipment.
References
Mamoudian, M., Mirkhani, H., Nehardani, Z. and Ghiaee, S., Synthesis and biological activity of two new calcium-channel blockers, mebudipine and dibudipine. J Pharm Pharmacol. 49:1229-1233, 1997.
Mirkhani, H., Omrani, G.R.,Ghiaee, S. and Mamoudian, M.,Effect of mebudipine and dibudipine, two new calcium channel blockers, on rat left atrium, rat blood pressure and human internal mammary artery. J Pharm Pharmacol, 617: 617-622, 1999
Sattari, S. and Jamli, F., High performance liquid chromatographic determination of cyclooxygenase II inhibitor rofecoxib in rat and human plasma. J Pharm Pharmaceut Sci, 3(3): 312-316, 2000
Gibaldi, M.; Perrier, D.; Pharmacokinetics. Marcel Dekker, New York and Basel, pp 45-109, 1982.
Mirfazaelian, A. and Mamoudian, M., A Comprehensive computer program for evaluation and teaching pharmacokinetic, XIIth Iranian Cong Physiol Pharmacol, Tehran, p 335, 1995.
Corresponding Author: M. Mahmoudian, Department of Pharmacology, Iran University of Medical Sciences, P.O. Box 14155-6183, Tehran, Iran. masmah99@iums.ac.ir
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps