J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(3):255-262, 2001
Pharmacologic Interventions in Nuclear Medicine Assessment of Cardiac Perfusion
Gilbert G. Matte1, David C. Barnes
Dalhousie University, Faculty of Medicine, Halifax, Nova Scotia, CanadaDouglas N. Abrams
University of Alberta, Faculty of Medicine, Edmonton, Alberta, CanadaManuscript received August 27, 2001, Revised October 17, 2001, Accepted November 21, 2001
PDF Version
Abstract
Drugs that are currently used for therapeutic purposes can also be used in diagnostic tests. This paper will review the use of such pharmacological interventions in cardiac assessment in Nuclear Medicine. To fully comprehend the effect of these drugs, a small review of diagnostic nuclear medicine as currently used to assess cardiac perfusion is included. This will allow pharmacists to understand the rationale behind the single administration of either vasodilator or inotropic agents and to review which drugs and food may interact with the test.
Introduction
Myocardial perfusion scintigraphy, a nuclear medicine technique, is one of the primary diagnostic techniques used to assess myocardial blood flow and function in patients with coronary artery disease. Following injection of an appropriate radiopharmaceutical, images acquired on a gamma camera provide information on both perfusion and contraction of the heart muscle. Treadmill exercise is the most commonly employed stressor in assessing these patient's hearts. Not all patients can exercise sufficiently to provide optimal test results, therefore pharmaceuticals that mimic the myocardial response to physical stress are often used.
Although diagnostic protocols vary among institutions, this paper provides an overview of how these studies are generally performed and how the information is acquired. Special emphasis will be given to the role of "pharmacologically induced stress" and the potential interactions that could occur with some common drugs and foods. These interactions can often necessitate diet restriction and cancellation of medication for patients prior to the test. This paper intends to summarize pertinent information that will allow a practising pharmacist to understand the procedure and properly inform patients referred for pharmacologically induced cardiac stress tests in nuclear medicine.
Assessment of Cardiac function
Assessment of chronic coronary artery disease (CAD) and myocardial perfusion are routinely performed by stress electrocardiography (ECG), angiography, ultrasound and nuclear medicine. In recent years, the sub-specialty of nuclear cardiology has been the most rapidly growing area in nuclear medicine, due to an increasing influence of the test results on patient management (1) and advances in new radiopharmaceuticals (2). Unlike other imaging modalities, which are used to evaluate anatomical aspects of the heart, cardiac studies in nuclear medicine are used to assess myocardial function.
The nuclear medicine technique assesses three aspects of heart function: blood flow within the heart muscle (myocardial perfusion), motion of the ventricular walls of the heart (segmental ventricular function) and the efficiency of pump function through calculation of the ejection fraction (global ventricular function).
Role of Stress in Myocardial Function Studies
A short review of the physiology of auto-regulation of coronary blood flow will allow a better understanding of the procedures. The auto-regulatory mechanism attempts to balance the supply and demand of oxygen for the heart. The heart rate, blood pressure, left ventricular contractility and left ventricular volume determine oxygen demand. The heart must consume more oxygen to do more work, but since it already extracts oxygen from the blood at nearly maximum efficiency at rest, it cannot simply increase its oxygen extraction efficiency as it works harder. An increase in oxygen demand stimulated by exercise must be accommodated through increased blood flow to the heart. Since blood flow to the left ventricle occurs mainly in diastole, the aortic diastolic pressure defines the coronary perfusion pressure. The required increase in blood flow must arise from a decrease in vascular resistance, not through an increase in blood pressure (3). Under normal conditions, the resistance to blood flow occurs mainly at the level of the small arteries and arterioles and at the myocyte cell walls. These small vessels are normally capable of dilating and dropping their resistance (4).
Patients without flow limiting stenosis, can augment myocardial blood flow by a factor of around 3 to 4 during exercise(4).
The difference between the coronary blood flow observed at rest and during maximal vasodilatation is defined as the coronary vasodilator reserve. In the presence of a coronary artery stenosis, perfusion pressure is reduced distally to the stenosis and coronary autoregulation can be exhausted resulting in transmural myocardial blood flow maldistribution and eventually ischemia(5).
Even if the patient is asymptomatic at rest, when the stenosis reaches sub-critical levels, as low as 30-45% of the vessel diameter, auto-regulation becomes incapable of sustaining the blood flow required under stress conditions (6,7). Most patients with moderate occlusion remain asymptomatic at rest. In the presence of severe coronary occlusion (greater than 85% of vessel diameter), however, the ability of the auto-regulatory mechanism to provide an adequate oxygen supply is impaired, even at rest (6,7). It is important to define the extent of ischemia of the heart in coronary artery disease in order to explain the patient's symptoms, assess risk and select therapy. Cardiac nuclear medicine allows in vivo monitoring of the blood supply to the different areas of the heart. While under stress, those areas of the heart supplied by normal vessels will receive an increase in blood supply while the tissues perfused by the obstructed arteries will not increase as much. The difference in blood perfusion to the affected areas induced by the stress can be detected and quantified using nuclear medicine techniques.
It is important that patients reach a high enough oxygen demand level during the stress procedure in order to exceed the coronary vasodilator reserve, otherwise the normal auto-regulatory response mechanisms may compensate sufficiently to mask significant stenosis. Upright aerobic or dynamic exercise (usually on a treadmill) using the large muscle groups stimulates the largest increase in coronary blood flow and cardiac output and remains the method of choice to induce stress for these studies. However, situations arise where it is not possible for patients to exercise sufficiently for optimal test results (Table 1). Alternatives designed to simulate the effects of exercise on the heart, such as cold pressor testing, have also been evaluated (8) but led to inconsistent results. Pharmacologic intervention has become the alternate method of choice.
Table 1. Contraindications to treadmill stressing
Conditions
General
Specific
Inability to sustain physical activities
degenerative joint disease
neuropathy
spinal cord injury
claudication
obstructive lung disease
liver or renal failureSpecific medication affecting response to exercise
Beta blockers
Verapamil
DiltiazemHeart Intrinsic conduction disease
Acute MI Uncontrolled
unstable angina
decompensated
Congestive heart failure
Uncontrolled hypertensionFrom Imaging Guidelines for Nuclear Cardiology Procedures 1996
Imaging Protocols
All nuclear medicine radiopharmaceutical myocardial perfusion agents, following i.v. injection, accumulate within the cardiac muscle, as a function of the blood flow to the area. The patient is then scanned under a camera and tomographic images are acquired. Images of the heart are reconstructed on a computer and the data can be displayed as slices of the heart or as a 3 dimensional representation of the whole heart. The data can be analyzed both quantitatively and qualitatively to define the level of perfusion to each area of the heart.
The first cardiac perfusion imaging protocols used 201Tl Thallium Chloride to evaluate myocardial blood flow. 201Tl Thallous Chloride was adopted because thallium mimics the biochemical and physiological distribution of potassium in the heart muscle. 201Tl has the most useful imaging characteristics of the thallium radionuclides available. Later 99mTc complexes such as Sestamibi and Tetrofosmine were developed to exploit the better imaging characteristics of 99mTc compared to 201Tl. 99mTc is bound to the molecule of either tetrofosmine or MIBI and decays with a physical half life of 6 hours by emission of a 140 Kev gamma ray. This particular photon energy can easily be detected by the standard gamma camera available in most hospitals. Although the 99mTc tracers are monovalent cations similar to potassium, they are distributed throughout the heart tissue by passive diffusion and do not mimic potassium or any naturally occurring molecule. Their exact mechanism of accumulation is still not fully understood but it has been suggested that 99mTc Sestamibi is trapped in the proximity of the mitochondria mostly due to its charge(2). Currently these three radiopharmaceuticals are the most commonly used. The distribution of these radiopharmaceuticals reflects the blood perfusion pattern within the heart muscle. After stress, an increase in perfusion is seen in normal tissue. More poorly perfused areas also accumulate the radiopharmaceutical but to a lesser extent when compared to the surrounding normal tissues. Typical myocardial perfusion procedures involve the acquisition of two sets of images following IV injection of the tracer. One set of images of the patient's heart is obtained with the patient in a resting state. A second set of images is obtained while the patient is under stress. The two sets of images are compared to obtain the diagnostic information.
Several imaging protocols, varying mostly in time between the stress and rest injections and injection dose (one day procedure, 2 days procedure, re-injection, etc.) are used. In order to illustrate the role of pharmaceutical induced stress, we will restrict our discussion to a widely accepted protocol which uses two injections of 99mTc Sestamibi on two separate days. Briefly, on the first day, the patient is stressed while being closely monitored by ECG. When the appropriate level of stress has been induced, 99mTc Sestamibi is injected into the patient where it rapidly accumulates in various tissues in proportion to blood flow.
One hour after injection the background activity will have cleared from the blood. The patient lies under a camera that rotates around them and acquires scintigraphic images representing the distribution of the radioactivity within the chest. On the second day, while at rest, the patient is injected a second time with 99mTc Sestamibi and imaged as before. By this time most of the radioactivity from the previous study has either decayed or been excreted by the patient.
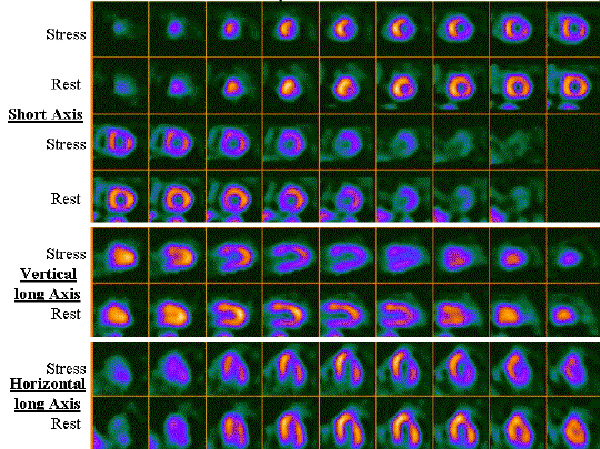
This image data can then be displayed in various formats to analyse tissue perfusion from different views. These computer reconstructions visualize the ventricular wall of the normal heart as an area of relatively intense radioactivity. The ventricular cavity is represented as an area of very low radioactivity. Areas of low radioactivity in the rest image of the heart wall indicate the presence and extent of an infarct or scar tissue in the heart muscle that cannot be salvaged. In contrast, the presence of an area of low radioactivity in the stress image can indicate either ischemic tissue (which can be salvaged) or infarcted or scar tissue. Comparing the stress and rest images will reveal areas of the myocardium that are perfused while at rest but not while under stress. These areas are at risk but amenable to further treatment. Figure 1 is a tomographic reconstruction of the heart of an individual both at stress and at rest.
Figure 1. Scintigraphic pictures at rest and following pharmacological intervention in a patient with an 80% block of two of the major coronary arteries, a diagonal branch of the left anterior descending (LAD) and the right coronary artery (RCA).
The rest images show normal perfusion, the stress images, however, show lack of perfusion in the anterior and lateral walls when compared to the rest images. The computer display presents three orientations of the heart. The first orientation is the short axis. The heart is oriented with its apex toward the viewer and the series of images in that row represent parallel, sequential images or slices of tissue starting at the tip of the apex and cutting across the left ventricle. The hole in all images represents the left ventricle cavity. The second and third representations are called vertical and horizontal long axis where the heart is oriented for the viewer to see both the apex and base of the left ventricle. In the vertical axis display the images are sliced from septum to lateral wall (and the horizontal axis displays the tissue with the slices from inferior to anterior wall).
Pharmacological stressing of the heart
Pharmacologic stress techniques can be used either in conjunction with exercise to enhance response in patients who can exercise only sub-optimally or used alone in patients who cannot exercise at all. The agents (Table 2) used can be divided into two groups: those that directly result in coronary vasodilation (adenosine and dipyridamole) and those that stimulate myocardial oxygen demand (dobutamine and arbutamine). Table 2 also lists the major side effects of the "stressing "drugs and their contraindications.
Table 2: Side effects and contraindications of the common pharmacologic stressors
Pharmaceutical
Dosage
(i.v. infusion)Mechanism
Side Effects (similar for all agents but different frequency)
Contraindications
Adenosine
0.140 mg/kg/min
0.84 mg/kg totalDirect Coronary Vasodilation
Asystole Myocardial infarction
Pulmonary edema
Ventricular tachycardia
Flushing
Chest pain
Dyspnea
ST-changes
Headache
Dizziness
Nausea and vomiting
Palpitations
Arm, back, shoulder pain
Parasthesia
ArrhythmiasOngoing wheezing
High grade atrioventricular block
Hypotension, 90mm Hg
Recent use of dipyridamole or xanthine.Dipyridamole
0.142 mg/kg/min
OR
0.568 mg/kg
OR
60 mg total doseIncrease Blood Adenosine Levels
Direct Coronary VasodilationTheophylline therapy
Active wheezing
High-grade atrioventricular blockDobutamine
0.04 mg/kg/min
Chronotropic
Inotropic
Beta agonistRecent MI
Unstable Angina
Left ventricular outflow tract obstruction
Critical aortic stenosis
Atrial tachyarrhythmias with uncontrolled ventricular response
Ventricular Tachycardia
Uncontrolled hypertension
Aortic dissections or large aortic aneurysmComposite from Imaging guidelines for Nuclear Cardiology Procedures 1996
Vasodilating Agents
Adenosine is a naturally occurring physiologic regulator (important in auto-regulation) of vascular blood flow. It acts on specific cell surface receptors by either slowing the heart rate (A1 receptors) or inducing vasodilatation (A2 receptors). It is synthesized intracellularly (vascular smooth muscle cells) but acts extracellularly. Free adenosine is rapidly reabsorbed into the cells where it is metabolised, therefore its physiological half-life is very short. Adenosine production is activated locally in the presence of myocardial ischemia. It increases oxygen supply through coronary vasodilatation. Although adenosine also directly decreases both heart rate and myocardial oxygen demand, the subsequent drop in blood pressure usually results in an increase in myocardial oxygen demand. Adenosine (140 ug/kg/min) given intravenously has a short half-life (<10 sec.), which allows for good control of both vasodilation and side effects.
Dipyridamole was first introduced in the late 1970's (9) and continues to be the most widely used agent in pharmacologic stress testing. Dipyridamole shares a common mechanism of action with adenosine, in that it indirectly increases the extra-cellular concentration of adenosine by blocking its cellular re-uptake. Dipyridamole increases cardiac output and heart rate while it decreases diastolic and mean blood pressure. The ejection fraction may increase slightly in normal patients but it will decrease slightly in coronary artery disease. The standard clinical dose is 0.14 mg/kg/min over 4 minutes and total dose of 0.56 mg/kg.
Both dipyridamole and adenosine increase coronary blood flow up to 2-5 times rest levels. Their effect is greater than that achieved with exercise. The response to both drugs is very fast and has some advantages over physical exercise. Dipyridamole's effects maximize approximately five minutes after infusion and last for 10 to 30 minutes, whereas adenosine's effects occur within two minutes and last only during infusion (10,11).
Certain drugs and food which interfere with the production or metabolism of adenosine can affect the test and need to be discontinued prior to the test. In the case of dipyridamole and adenosine, methylxantines are typical of agents interacting with the binding of adenosine to its receptors. They are listed in Table 3. Table 4 lists the common sources of the most ubiquitous methylxantines.
Table 3: Pharmaceuticals to be discontinued before pharmacologic stress
Pharmaceutical
Rationale
Stress with Adenosine and Dipyridamole
Caffeine
competitive inhibition
Theophylline
competitive inhibition
Aminophylline
competitive inhibition
Pentoxifylline (Trental)
competitive inhibition
Nitroglycerin
limits effect
Stress with Dobutamine
Beta blocker
competitive inhibition
Calcium channel Blockers
Limits effects
Table 4: Common sources of caffeine and theophylline
Hot Beverages
Coffee, Tea, Cocoa, Hot Chocolate
Cold Beverages
Cola drinks i.e. Coke, Pepsi and others, Other Soft Drinks i.e. Tab, Mountain Dew, Mello Yellow
Foods
Generally limited to foods containing caffeine mostly chocolate
Drugs Containing Caffeine
Anacin, Cafergot, Darvon, Fiorinal, Wigraine
Drugs Containing Theophylline
Aerolate
Elixophyllin SR
Respbid
Sustaire
Theophylline SR
Theochron
Uniphyl
Bronkodyl S-R
LaBID
Slo-Bid Gyrocaps
Theo-24
Theospan SR
Theolair-SR
Constant-T
Lodrane
Slo-Phyllin Gyrocaps
Theoclear L.A.
Theobid (Jr.) Duracaps
Theo-Time
Duraphyl
Quibron-T S/R
Somophyllin-CRT
Theovent Long Acting
Theo-Dur and Sprinkle
Theophyl-SR
Positive Inotropic Agents
Positive inotropic agents, such as dobutamine and arbutamine, affect the force of the muscular contractions of the heart. They are usually used in a patient who cannot exercise adequately and has contraindications to dipyridamole or adenosine.
Positive inotropes increase myocardial oxygen demand by stimulating beta receptors in the heart. The resultant increase in blood flow is in the order of two to three times rest levels. Their increase in blood flow is comparable to exercise but is less than that observed with dipyridamole or adenosine. They are considered to be the last line of stress testing as they do not produce as high a peak heart rate as physical exercise and do not yield exercise duration and capacity information. Therefore, they are used only in patients who cannot exercise and are contraindicated to dipyridamole and adenosine (12, 13).
Patients with wheezing (requiring theophylline), patients with hypotension at baseline, and patients with high-grade atrioventricular block (without pacemaker) are contraindicated to dipyridamole studies. Inotropic agents are selected over vasodilatators for these patients.Patients with recent unstable angina or recent myocardial infarction should be carefully screened.
Dobutamine hydrochloride has a rapid onset of action (1 to 2 minutes i.v.). It increases stroke volume, cardiac output and inotropy substantially at low doses (5 to 20 g/kg/min) but only modestly increases heart rate. Both heart rate and systolic blood pressure increase incrementally with dose. Coronary vascular resistance decreases distal to the stenosis but resistance increases at the stenotic site. The presence of coronary artery disease decreases the flow response to dobutamine relative to normal patients.
Arbutamine is similar to dobutamine with respect to inotropic and chronotropic activity, but is a mixed beta-1 and beta-2 agonist with mild alpha-1 affinity in contrast to the strong beta-1 but weak beta-2 and alpha-1 properties of dobutamine (14).
Table 3 lists the drugs that need to be stopped to prevent possible interference with the pharmacological action of Dobutamine. These include mostly beta blockers and calcium channel blockers; both decrease the response induced by the inotropic agents.
Pharmacologic Stress Protocols
Pharmacologic stress is often combined with some form of physical exercise as evidence indicates this improves blood flow, tracer uptake and image quality while it decreases the incidence of side effects from dipyridamole and adenosine (15).
Dipyridamole
With the dipyridamole protocol, dipyridamole is usually administered as an i.v. infusion at a rate of 0.14 mg/kg/min (total dose of 0.56 mg/kg or 60 mg) over 4 minutes. The radiotracer is injected when maximum vasodilatation is reached, usually after 3 minutes, which generally occurs approximately seven minutes from onset of infusion. When physical exercise is included in the protocol, it is generally initiated two minutes prior to injection of the tracer (13).
Non cardiac side effects are commonly reported with dipyridamole use, including headache (12%) and dizziness, nausea, vomiting and flushing (12%) while cardiac related side effects such as chest pain (20%) and ST depression (7.5%) are less severe than with exercise stress (16). The risk of side effects increases with higher doses. Side effects can be easily reversed by administration of an i.v. bolus of 50 to 75 mg aminophilline or an infusion of 250 to 500 mg over 20 minutes. Nitroglycerin sublingual can be administered the rare time aminophylline is ineffective. Severe side effects are rare with non-fatal myocardial infarction estimated to occur in 0.1% of patients (17). A list of contraindications to vasodilator induced stress testing are given in Table 3.
Adenosine
Due to the short half-life of adenosine, its use differs from dipyridamole. The radiotracer is injected around three minutes after beginning the infusion (140 g/kg/min) which lasts for six minutes (13).
In one study, side effects including chest, throat and jaw pain, headache and flushing and ischemic electrocardiogram changes were observed in up to 83% of patients. They include headaches (85%), flush (29%), chest, throat or jaw pains (57%) (18). Fortunately these side effects are readily and immediately reversed by termination of the infusion when necessary. Aminophylline and Esmolol can be useful to reverse the effects of adenosine but the need for their use is rare.
Dobutamine
A standard dobutamine protocol starts at a low dose, in the order of 5 ug/kg/min and increments every three minutes to 10, 20, 30 and finally 40 ug/kg/min. The radiopharmaceutical is injected two to three minutes prior to the end of the highest dose level. (13). In some laboratories, atropine is used to help reach the desired heart rate with dobutamine. This latter protocol is similar except that 0.5 mg of atropine is injected approximately 9 minutes after the beginning of the dobutamine infusion (19).
Dobutamine increases heart rate, systolic blood pressure and the rate pressure product in patients. Although serious side effects are rare, minor effects are common, occurring in up to 75% of cases in one study (20). These included typical (26%) and atypical (5%) angina, palpitation (29%), flushing (14%), headache (14%) and dyspnea (14%). Other side effects include hypotension (3.4%), supraventricular (4.4%) and ventricular tachycardias (3.8%)(20,21). Table 3 lists the drugs required to be discontinued prior to using dobutamine.
Fig. 1 shows the images at rest and stress of a 65 y.o. male who gives vague history of intermittent upper sternal burning, usually provoked by effort, relieved by rest. The post dipyridamole images show a decrease in the anterior and lateral walls but no corresponding defect on the rest images. This demonstration of decreased coronary flow reserve in response to pharmacologic stimulation is due to 80% stenosis of two of the major coronary arteries, a diagonal branch of the Left anterior descending-LAD and the Right coronary artery-RCA.
Pharmacologic Enhancement of Myocardial Viability
There is some evidence to suggest that defects observed on the rest images in some patients overestimates the extent of irreversibly damaged myocardium. A second method to improve the differentiation of irreversibly damaged tissue from poorly perfused but still viable tissue exploits the vasodilating effects of nitrates such as nitroglycerin and isosorbide dinitrate. The rationale for their use is that nitrates also induce venous dilation as well as the epicardial arteries, including the stenosis site and may enhance collateral perfusion distal to the occlusion. Therefore the administration of a nitrate to the patient before injection of the radiopharmaceutical during the rest image may enhance uptake of radiopharmaceutical in the hypoperfused tissue which would increase detection of patients having treatable cardiac defects (22). Both nitroglycerin (23) and isosorbide dinitrate (24) can be used. Nitroglycerin (0.4 mg) is given sublingually and the radiopharmaceutical injected approximately five minutes after the appropriate hemodynamic response (blood pressure and heart rate) is observed. The isosorbide dinitrate protocol involves an infusion of the nitrate (10 mg in 100 mL saline) over 20 minutes and upon reaching the appropriate drop in blood pressure, the radiopharmaceutical is injected. The drop in blood pressure is expected and monitored. Sildenafil is reported to have a significant effect. Concomittant administration is contra-indicated (25). The likelihood for sildenafil to be administered prior to a major Nuclear Medicine exam is both limited and unlikely. Regardless, it cannot be ignored and patients should be properly counselled. Most protocols include installation of an IV line prior to the test to maintain intravenous access and fluid administration if needed.
Pharmacists role
All nuclear medicine departments send information to patients when the appointment for the test is confirmed which includes restriction in both food and drugs. The patient, however, is not always familiar with either the procedures or the reasoning behind the interruption of some of the current medication or common food. The community pharmacist's role becomes essential in both reassuring and helping patients to understand the procedure and its requirement. Hospital pharmacists monitoring patient therapy will also be able to understand therapy interruption and advise patients accordingly.
Conclusion
Pharmacological myocardial stressing is a common technique used extensively to assess heart perfusion. The diagnostic test can use a variety of fast acting vasodilators such as dipyridamole and adenosine or positive inotropes such as dobutamine depending on the patient. Their administration in these tests differs from their usual therapeutic use. This review should ensure pharmacists are familiar with the procedures, the drugs used during the stressing as well as the drugs that could interfere with the stressing procedures.
References
Meyers, A. and Wintch, K., A retrospective comparative study of changes in nuclear medicine cardiac stress testing. J Nucl Med Technol 25: 275-278, 1997.
Jain, D., Technetium-99m labelled myocardial perfusion imaging agents. Semin Nucl Med 24:221-236, 1999.
Braunwald, E., Sarnoff, S.J. and Case, R.B. et al, Hemodynamic determinants of coronary flow: Effect of changes in aortic pressure and cardiac output on the relationship between myocardial oxygen consumption and coronary flow. Am J Physiol 192: 157-163, 1958.
Holmberg, S., Serzysko, W. and Varnauskas, E., Coronary circulation during heavy exercise in control subjects and patients with coronary heart disease. Acta Med Scand 190(6):465-80, 1971.
Rouleau J, Boerboom LE, Surjadhana A, Hoffman JI. The role of autoregulation and tissue diastolic pressures in the transmural distribution of Left ventricular blood flow in anesthetized dogs. Circ Res 1979 Dec; 45(6) :804-815.
Gould, K.L, Lipscomb, K. and Hamilton, G.W., Physiologic basis for assessing critical coronary stenosis. Am J Cardiol 33:87-94, 1974.
Gould, K.L., Lipscomb, K. and Calvert, C., Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation 51(6):1085-94, 1975.
Rodger, J.C., Railton, R., Parekh, P. and Newman, P., Effect of cold stimulation on myocardial perfusion. An investigation using thallium-201 scintigraphy. Br Heart J 52(1):57-62, 1984.
Gould, K.L., Noninvasive assessment of coronary stenoses by myocardial perfusion imaging during pharmacological coronary vasodilatation. I. Physiological basis and experimental validation. Am J Cardiol 41:267-277, 1978.
Marchant, E., Pichard, A.D. and Casanegra, P. et al, Effect of intravenous dipyridamole on regional coronary blood flow with 1-vessel coronary artery disease: Evidence against coronary steal. Am J Cardiol 53:718-721, 1984.
Wilson, R.F., Wyche, K. and Christensen, B.V. et al, Effect of adenosine on human coronary arterial. Circulation 82:1595-1606, 1990.
Travin, M.I. and Wexler, J.P., Pharmacological Stress Testing. Semin Nucl Med 24(4): 298-318, 1999.
Garcia, E.V., editor, Imaging guidelines for Nuclear Cardiology procedures. J Nucl Cardiol 3: G4-G45, 1996.
Young, M., Pan, W. and Wiesner, J. et al, Characterization of arbutamine: a novel catecholamine stress agent for diagnosis of coronary artery disease. Drug Dev Res 32:19-28, 1994.
Pennell, D.J., Review Pharmacological cardiac stress: when and how? Nucl Med Commun 15:578-585, 1994.
Ranhosky, A. and Kempthorne-Rawson, J., and the intravenous Dipyridamole Thallium Imaging study group. The safety of intravenous dipyridamole thallium perfusion imaging. Circulation 81:1205-1209, 1990.
Lette, J., Tatum, J.L. and Fraser et al., Safety of dipyridamole testing in 73,806 patients. The multicenter dipyridamole safety study. J Nucl Cardiol 2:3-17, 1995.
Verani, M.S., Mahmarian, J.J. and Hixson, J.B., Diagnosis of coronary artery disease by controlled coronary vasodilation with adenosine and thallium-201 scintigraphy in patients unable to exercise. Circulation 82:80-87, 1990.
Caner, B., Karanfil, A. and Uysal, U. et al, Effect of an additional atropine injection during dobutamine infusion for myocardial SPET. Nucl Med Commun 18:567-573, 1997.
Hays, J.T., Mahmarian, J.J. and Cochran, A. et al, Dobutamine thallium-201 tomography for evaluating patients with suspected coronary artery disease unable to undergo exercise or vasodilatator pharmacologic stress testing. J Am Coll Cardiol 21:1583-1590, 1993.
Elhendy, A., Valkema, R. and Van Domburg, R.T. et al, Safety of dobutamine-atropine stress myocardial perfusion scintigraphy. J Nucl Med 39:1662-1666, 1998.
He, Z.X., Verani, M.S. and Liu X.J., Nitrate-augmented myocardial imaging for assessment of myocardial viability. J Nucl Cardiol 2:352-357, 1995.
Flotats, A., Carrio, I. and Estorch, M. et al, Nitrate administration to enhance the detection of myocardial viability by techenetium-99m tetrofosmin Single-Photon Emission Tomography. Eur J Nucl Med 24:767-773, 1997.
Sciagra, R., Bisi, G. and Santoro, G.M., et al, Comparison of Baseline-Nitrate Technetium-99m Sestamibi with Rest-Redistribution Thallium-201 Tomography in Detecting Viable Hibernating Myocardium and Predicting Postrevascularization Recovery. J Am Coll Cardiol 30:384-391, 1997.
Webb, D.J., Muirhead, G.J., Wilff, M., et al, Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol 36(1):25-31, 2000.
Corresponding Author: Gilbert G. Matte, Department of Diagnostic Imaging, QEII Health Sciences Centre, VG Site, 1278 Tower Road, Halifax, Nova Scotia, Canada. gmatte@is.dal.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps