J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 4(1):7-14, 2001
Investigation of interpolymer complexation between Carbopol and various grades of polyvinylpyrrolidone and effects on adhesion strength and swelling properties
Yvonne Tze Fung Tan1, Kok Khiang Peh, Othman Al-Hanbali,
School of Pharmaceutical Sciences, University of Science Malaysia, Minden, Penang, Malaysia.Received November 22nd, 2000, Revised February 22nd, 2001, Accepted February 23rd, 2001
ABSTRACT
Purpose
. To investigate the interpolymer complexation between Carbopol 934P (CP) and various grades of polyvinylpyrrolidone (PVP) (K90, K32, C15, and VA/S-630).Methods. Amount of fresh and dried CP-PVP complexes, water retaining capacity, apparent density, pH, conductivity, FTIR, swelling and adhesion strength were studied
Results. Interpolymer complexation occurred between CP and all the PVP, but most significantly with PVP K90. Maximum amount of fresh and dried CP-PVP K32 complexes were obtained at a weight ratio of 1:1. On the contrary, CP concentration was linearly related to amount of CP-PVP K90 complexes produced and their water retaining capacity were all above 97%. Increase in CP concentration caused a decrease in pH, but an increase in conductivity for all the CP-PVP complexes. The apparent density of the filtrate of CP-PVP K90 complex was the lowest and its IR spectrum was similar to that of pure PVP K90, indicating that all the CP has interacted with the PVP K90. Discs of physical mixtures of CP-PVP K90 swelled gradually and reached a maximum after 20-30 hr, while discs of solid complex swelled readily and reached a maximum within 20 hr. Adhesion strength was directly correlated to CP content. However, adhesion strength of solid CP-PVP K90 complex was lower than the physical mixture of the pure polymers.
Conclusion. Interpolymer complexation occurred between CP and PVP but to a different extent for the various grades of PVP. Complexation was most prominent between CP and PVP K90.
INTRODUCTION
The phenomena of interpolymer interactions have been the focus of intensive fundamental and applied research (1). Studies have been carried out on many different polymer blends and types. Such combinations may possess unique properties that are different from those of individual component. There is a great potential in utilizing these blends in many pharmaceutical preparations especially in controlled release drug delivery systems. Elgindy and Elegakey (2) prepared PVP-Carbopol (934, 940, and 941) complexes and demonstrated their use in the development of sustained-release products. Satoh et.al. (3) reported that the use of a 3:2 weight ratio of hydroxylpropylcellulose and carboxylvinyl polymer as excipients in tablets significantly decreased the bioadhesion force compared to that of the parent polymers. Similar effects were noted with compressed tablets prepared using chitosan and sodium hyaluronate (4). Recently, the interaction of carboxylvinyl polymer with hydroxylpropylcellulose and sodium carboxymethylcellulose and its effect on the bioadhesive strength and release of verapamil have been reported (5). Anlar et al (6) prepared buccoadhesive morphine sulfate tablet containing hydroxypropyl methylcellulose and carbomer and found the two polymers interacted in acidic pH medium. Complexes of polyvinylpyrrolidone and polyvinyl acetate phthalate serving as a stable, enteric polymer was investigated by Kumar et.al (7).
Various methods have been used to investigate interactions between polymers. Measurements of turbidity, pH and ionic strength as a function of weight ratio of polymer in the media (8), viscosity (9), infrared spectroscopy, NMR, thermal analysis, pKa, powder X-ray diffraction (6) were employed to evaluate interpolymer complexation. Nevertheless, a comprehensive investigation of interactions between carbomer and various grades of polyvinylpyrrolidone has not yet been reported.
The aim of the present study was to investigate the interaction between Carbopol 934P (CP), a widely used polymer for the preparation of buccal bioadhesive gel, and different grades of polyvinylpyrrolidone (PVP) (K90, K32, C15, and VA/S-630), which were characterized by their molecular weight and viscosity in solution expressed as a K-value. The interaction was examined using parameters such as amount of fresh and dried complexes formed, water retaining capacity, apparent density, pH, and conductivity. In addition, the CP-PVP complexes were analyzed by FTIR spectroscopy and subjected to swelling and adhesion strength tests.
MATERIALS AND METHODS
Materials
Carbopol 934P (CP) (polyacrylic acid cross-linked with allyl sucrose) was purchased from B.F. Goodrich, Cleveland, OH, USA. Various grades of polyvinylpyrrolidone (PVP) (linear polymers of 1-vinylpyrrolidine-2-one) (K90, K32, C15, and VA/S-630) were supplied by ISP Technologies Inc., Wayne, NJ, USA. The typical molecular weight for PVP K90, K32, C15, and VA/ S-630 was 1300000, 58000, 8000 and 58000, respectively. The typical viscosity for PVP K90, K32, C15, and VA/S-630 was 55, 2.5, 1.5 and 2.5 mPa.s, respectively. The chemical structures of CP and PVP are shown in Figure 1. All other chemicals were reagent grade and used as received.
Figure 1: Chemical structures of (a) CP and (b) PVP
Preparation of Polymer Complex
2% aqueous stock dispersions of CP and PVP K32 were first prepared. Appropriate amounts of CP and PVP dispersions were mixed to obtain CP to PVP weight ratios of 3:7, 1:1, and 7:4 (weight fraction of 30%, 50% and 64% of CP). Total polymer concentration was fixed at 2% by weight in each sample. The two polymeric solutions were stirred uniformly for 1 hour and filtered. The residue consisting of CP-PVP complex was collected and weighed to represent the weight of fresh polymer complex (Wf). The complex was subsequently dried at 110 °C until a constant weight (Wd) was obtained. The dried CP-PVP complex was ground and sieved through a 60 mesh (250µm) sieve, while the filtrate was collected and kept for further analysis. The water retaining capacity of the complex is defined as (Wf - Wd)/ Wf expressed as a percentage.
pH and Conductivity Measurement
The pH and conductivity of CP and PVP (K90, K32, C15, VA/S-630) mixtures at various weight ratios were measured using a pH meter and a conductivity meter with a cell constant of 1 cm–1 at 25°C (HORIBA DS-32E, Kyoto, Japan), respectively. Total polymer concentration was fixed at 2% by weight in each sample.
Measurement of Apparent Density
The apparent densities of 2% of the pure polymer dispersions (CP or PVP) and the filtrates of CP-PVP (K90, K32, C15, and VA/S-630) complexes (1:1) were determined using a pycnometer at 25°C. Each measurement was run in triplicate.
FTIR measurement
FTIR spectra of CP, PVP K90 and CP-PVP K90 (1:1) solid complex were measured using KBr pellet method (Perkin-Elmer 2000, Germany) at 25°C.
Preparation of CP-PVP Discs for Swelling and Adhesion Studies
Discs (13 mm diameter) comprising 200 mg of CP-PVP K90 solid complex or physical mixtures at CP to PVP weight ratio of 1:1, were prepared using an IR hydraulic press, at a pressure of 9 tonnes and compression time of 30 s.
Swelling Studies
The CP-PVP K90 disc was submerged in a glass petri dish containing 70 ml of phosphate buffer solution. Increase in diameter was measured at different time intervals over 70 hr with a vernier caliper at 25° C. Each test was carried out in triplicate.
Adhesion Strength Measurement
The adhesion strength of the CP-PVP K90 disc was determined using a Texture Analyzer equipment (TA-XT 2, Stable Micro Systems, Surrey, UK) equipped with a 5 kg load cell. The inverted surface of chicken pouch, after removal of its contents and surface fats, was used as the test membrane. The chicken pouch was first stored frozen in a simulated saliva solution (2.38g Na2HPO4, 0.19g KH2PO4 and 8.00g NaCl in 1000 ml of distilled water adjusted to pH 6.75) and thawed to room temperature before use. The chicken pouch was mounted onto a cylindrical perspex support of 2 cm diameter and 4 cm length. A double-sided foam tape was placed underneath the chicken pouch on the perspex support at the cross-sectional end to provide cushioning effect. The chicken pouch was further secured by placing a perspex ring over the perspex support. The whole perspex support was positioned at the bottom of the measuring system and held in place by a clamp. The disc sample was affixed to another perspex support of similar dimension and screwed onto the upper probe of the instrument. The two perspex supports were aligned to ensure that the disc came into direct contact with the exposed surface of the chicken pouch.
During measurement, 150
ml of simulated saliva solution was evenly spread onto the surface of the test membrane. The upper perspex support was lowered at a speed of 1 mm/s until contact was made with the membrane, at a preset force of 0.5 N for a contact time of 120 s. The support was then withdrawn at a speed of 1 mm/s to a distance of 5 mm. An acquisition rate of 200 points/s was chosen for the analysis. Data collection and calculation were performed using the XTRA Dimension software package of the instrument. Two parameters, namely, work of adhesion and peak detachment force were determined. The work of adhesion was calculated from the area under the force-distance curve, and the peak detachment force was taken as the maximum force needed for detaching the film from the membrane. Measurements were performed in ten replicates. All the measurements were conducted at 28° C and relative humidity of 50-60%.Statistical analysis
The results obtained from the experiments were analyzed statistically using multivariate tests. A statistically significant difference was considered when p<0.05. When a statistically significance difference was found, Tukey HSD (honestly significant difference) test was then conducted. The results were also subjected to bivariate correlation statistical treatment. Regression polynomial was calculated using SPSS statistical software and was applied to approximate the response surface and contour plots for the swelling test using the software Mathematica.
RESULTS AND DISCUSSIONS
In general, the amount of fresh complexes and water retaining capacity were positively correlated with the molecular weight and viscosity type of PVP, while the solid complexes and apparent density of the complex filtrates were negatively associated with the molecular weight and viscosity type of PVP, for the various CP-PVP (C15, K32, K90 and VA/S630) complexes at a weight ratio of 1:1. When CP-PVP K90 complexes was investigated as a function of CP concentration, the amount of fresh and solid complexes obtained, water retaining capacity, conductivity, adhesion parameters and the disc swelling of physical polymer mixture were directly correlated to CP concentration. These results were substantiated by the Pearson’s correlation coefficients, at a significance level of at least 0.05.
Water retaining capacity
It has been reported by Gupta et al. (5) and Takayama and Nagai (8) that the maximum intermolecular complexation between CP and PVP was at a ratio of 1:1. In order to confirm this, we started off with 3 different CP to PVP K32 weight fractions, including values lower and higher than 1:1. Table 1 shows the amount of fresh and dried CP-PVP K32 complexes formed from a series of CP to PVP K32 weight ratios. The amount of fresh and dried complexes augmented with an increase in CP concentration up to a weight ratio of 1:1 (50% CP), but decreased with a further increase in CP concentration.
Table 1: The amount of fresh and dried CP-PVP complexes formed*
/Y.Tan/Table1.gif)
This indicated that maximum CP-PVP K32 complexation was at a weight ratio of 1:1, similar to the findings as previously reported. When we continue our study using different grades of PVP (K90, C15, S-630) at a fixed weight ratio of 1:1, the amount of fresh CP-PVP complexes obtained was highest for K90, followed by VA S630, K32 and C15. On the contrary, the weight of the dried CP-PVP solid complexes, at a fixed weight ratio, were not significantly different (p>0.05) for the various PVP employed. Tukey’s HSD test indicated that at a fixed weight ratio, all the solid complexes belonged to one homogeneous subset.
Since the fresh CP-PVP K90 complex was significantly higher than complexes formed with the other grades of PVP, the effect of CP concentration on interpolymer complexation was carried out. It was found that in contrast to the CP-PVP K32 complex, when CP increased from 30 to 80%, the amount of fresh CP-PVP K90 complex also increased. Fig. 2 shows the water retaining capacity as a function of various CP-PVP complexes.
Figure 2: Water retaining capacity as a function of CP-PVP complexes
At a fixed weight ratio of 1:1, water retaining capacity was highest for K90, followed by VA S630, K32 and C15. However, it is interesting to notice that the water retaining capacity of CP-PVP K90 complexes were all above 97%, even though the weight fraction of CP increased from 30 to 80%. This indicated that PVP K90, which has a high viscosity and molecular weight, played an important role in the complexation process, whereas CP concentration determined the extent of complexation with the rest of the PVP grades studied. It is worth noticing that although PVP VA/S630 and PVP K32 have similar viscosity value and molecular weight, the water retaining capacity of CP-PVP VA/ S630 was more superior than CP-PVP K32, which may be contributed by the presence of vinyl acetate in the former polymer.
Effect on apparent density
Figure 3 shows the apparent density values of PVP (K90, S-630, K-32 and C-15) solutions and CP-PVP (K90, S-630, K-32 and C-15) filtrates.
Figure 3: Apparent density as a function of CP-PVP complexes
There was no significant difference (p>0.05) in the apparent density values among the various PVP solutions. However, a statistically significant difference was obtained among the apparent density values of the various CP-PVP filtrates (p<0.05). The filtrate of CP-PVP K90 complex possessed the lowest apparent density among all the filtrates. Post hoc test showed that there were 2 subsets, with the apparent density of CP-PVP K90 as one subset and the apparent density of the remaining CP-PVP complexes in another subset. In addition, the apparent density values of the CP-PVP (S-630, K-32 and C-15) filtrates were higher than their respective PVP solutions.
Effect on pH
At 25° C, a 2% dispersion of CP, PVP K90, PVP K32, PVP C15 or PVP/VA S630 has a pH value of 2.8, 3.77, 3.44, 4.9 and 3.5, respectively. Aqueous dispersions of CP exhibited pH values between 2.8 to 3.2, depending on its concentration. The greater the concentration, the higher the carboxyl concentration and hence the lower the pH. In general, increase of CP concentration caused a decrease in pH for all the various PVP (Fig 4). All the CP-PVP complexes were insoluble in acidic aqueous solution, but when the pH was adjusted to 7 with 2M sodium hydroxide, decomplexation took place.
Figure 4: pH as a function of CP-PVP complexes
Effect on Conductivity
Since conductivity is based on the movement of ions, it follows that the concentration of ions in the solution will have a great bearing on the assessment of ions in the solution. Therefore, conductivity can be used to indicate and estimate the degree of complexation between polymers in aqueous solution. All grades of PVP interacted with CP and conductivity was positively correlated to CP concentration (Fig. 5). However, these conductivity values were relatively low when compared to the pure CP aqueous dispersion.
Figure 5: Conductivity as a function of CP-PVP complexes
A marked increase in conductivity was observed for CP-PVP K90 complex when the CP was increased from 60 to 90%, while below 60% of CP, there was only a gradual increase similar to the rest of the CP-PVP complexes. This may be explained that at a lower CP concentration, the insoluble complex was surrounded by poor conducting PVP solution. At a higher CP concentration, a larger amount of flocculated insoluble complexes were obtained, providing a conductive pathway for the flow of electrons and thus increased the conductivity. The presence of molecules rather than ions in organic compounds explained why aqueous solutions of PVP and most organic compounds and organic liquids did not conduct current. This was in contrast to the conductivity of inorganic acids, bases and salts, which are strong electrolytes (10).
FTIR spectral analysis
Since the complexation was most prominent between CP and PVP K90, further studies including FTIR spectral analysis, adhesion strength and swelling studies were carried out.
The FTIR spectra of CP, PVP-K90 and dried CP-PVP K90 (1:1) complex are given in Figure 6.
Figure 6: FTIR spectra of (a) CP, (b) PVPK90 and (c) CP-PVPK90 complex (1:1)
From these plots, the characteristic broad hydroxyl group (O-H) stretching absorption peaks at 3117 cm-1 and 3437 cm-1 for CP and PVP K90 respectively, were conspicuously less or absent in the infrared spectrum of the CP-PVP K90 complex. The characteristics absorption peaks at 1642 cm-1 and 1712 cm-1 due to the carbonyl groups for the PVP-K90 and carboxyl groups of CP, appeared to be less sharp and shifted to 1698 cm-1 in the complex. The difference in IR spectrum of the complex was attributed to the hydrogen bonding formation between the carboxyl groups of CP and carbonyl groups of PVP.
The FTIR spectra of the dried filtrates of the CP-PVP K90 complexes (at 30, 50 and 80% CP) were found to be similar to that of pure PVP K90. Therefore, it was concluded that all the CP has interacted with the PVP K90. But the spectra of the dried filtrates of CP-PVP (K-32, C-15and S-630) exhibited broad bands having the characteristics of both CP and PVP, which indicated that not all the CP has interacted with PVP.
Effect on Swelling
Regression polynomials for the disc swelling of CP-PVP K90 physical powder mixture and CP-PVP K90 solid complexes were calculated and applied to approximate the response surfaces and contour plots.
The final models for the disc swelling of CP-PVP K90 powder and spongy complexes are as follows:
Disc swelling of powder complex = 1.706+0.09478x1+0.00265x1x2-0.00625x2-0.002626x12-
0.00006585x12x2+0.0000003939x12x22+0.00004714x13+
0.0000001667x13x2-0.0000002436x14-0.000000191x23x1+0.000000005086x24
(r2 = 0.933)Disc swelling of spongy complex = 2.696+0.199x1+0.002519x1x2-0.0139x2-0.01293x12-
0.0000639x12x2+0.00000037x12x22+0.0002747x13+
0.0000001634x13x2-0.000001784x14-0.0000001601x23x1+0.00000000697x24
(r2 = 0.764)Where x1 = time (hr),x2 = weight ratio of CP (% CP)
Figure 7 shows the corresponding response surface and contour plot of the swelling profile for discs prepared from physical powder mixtures of CP and PVP K90.
Figure 7: Response surface and contour plot of the swelling profile for discs prepared from physical powder mixtures of CP and PVP K90.
Swelling increased gradually at a constant rate and reached the maximum after 20-30 hr. Subsequently, it was maintained at the maximum for the rest of the study. For the initial 20 hours, an increase in CP did not provide a significant difference in the swelling profile. However, between 20-30 hours, disc with a lower % of CP needed a longer time to reach the maximum swelling.
In contrast, the swelling profile of discs consisting of the solid complex hydrated rapidly and reached maximum values after 10-20 hr, followed by a decline in swelling with the passage of time (Fig 8).
Figure 8: Response surface and contour plot of the swelling profile for discs prepared from solid CP-PVP K90 complex.
In addition, the swelling profile of solid complexes appeared to be closely similar and independent of the ratios employed. This behavior was mainly attributed by the tendency of the solid complex to disperse readily in the aqueous medium, indicating a possible loss of controlled release property of the polymer complex.
Effect on Adhesion Strength
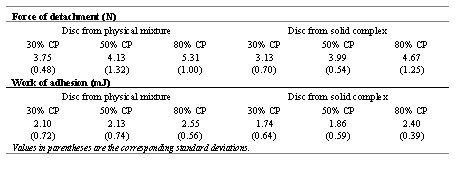
Table 2 shows the peak detachment force (Df) and work of adhesion (Wadh) to the chicken pouch membrane, for discs prepared from both the physical mixtures and solid complexes of CP-PVP K90 (1:1).
Table 2: Results from adhesion strength study using CP and PVP K90
It was found that Df and Wadh were linearly related to CP content. An increase in CP% caused an increase in Df and Wadh. On the other hand, a significant decrease in Df and Wadh were obtained between discs prepared from physical mixtures and solid complexes of CP-PVP K90, at a fixed CP% (p<0.05).
A bioadhesive product designed for application to the oral cavity should possess adhesive properties, since this can enhance the contact time of the product at the site of application and hence improve the clinical efficacy. CP was described as a bioadhesive polymer and exhibited concentration dependent adhesive interactions with substrate (11). Increasing CP934P concentration was found to enhance significantly the adhesiveness in bioadhesive gel formulation which was also reported in our previous study (12). It is worth noticing that disc formulated from solid complex of CP-PVP K90 possessed lower adhesion strength, as compared to disc formulated with physical powder mixture. Interpolymer complexation between CP and PVP K90 seemed to suppress interaction between molecules of polymers and tissue membrane, leading to a reduction in the adhesion force.
CONCLUSION
Interpolymer complexation occurred between CP and PVP, but to a different extent for the various grades of PVP. Complexation was most prominent between CP and PVP K90, possibly due to the higher viscosity and molecular weight of PVP K90. In addition, the swelling and adhesion properties of the CP-PVP K90 solid complex were found to differ from the physical mixture of pure polymers.
ACKNOWLEDGEMENTS
The authors wish to thank the University of Science Malaysia, Penang, Malaysia, for providing the IRPA research grant in support of this work.
REFERENCES
Shojaei, A. H., Buccal mucosa as a route for systemic drug delivery: a review. J Pharm. Pharmaceut. Sci. 1 (1): 15-30, 1998.
Elgingy, N. A., and Elegakey, M. A., Carbopol-polyvinylpyrrolidone flocculation. Pharm. Sci. 49: 427-434, 1981.
Satoh, K., Takayama, K., Machida, Y., Suzuki, Y., Nakagaki, M., and Nagai, T., Factors affecting the bioadhesive property of tablets consisting of hydroxypropyl cellulose and carboxylvinyl polymer. Chem. Pharma. Bull. 37(5): 1366-1368, 1989.
Takayama, K., Hirata, M., Machida, Y., Masada, T., Sannan, T., Nagai, T., Effect of interpolymer complex formation on bioadhesive property and drug release phenomenon of compressed tablet consisting of chitosan and sodium hyaluronate. Chem. Pharm. Bull. 38: 1993-1997, 1990.
Gupta, A., Garg, S., and Khar, R.K., Interpolymer complexation and its effect on bioadhesive strength and dissolution characteristics of buccal drug delivery systems. Drug Dev. Ind. Pharm., 20 (3): 315-325, 1994.
Anlar, S., Capan, Y., Guven, O., Gogus, A., Dlakara, T., and Hincal, A. A., Formulation and in-vitro-in-vivo evaluation of buccoadhesive morphine sulfate tablets, Pharm. Res., 11:231-236, 1994.
Kumar V., Yang, T., and Yang, Y., Interpolymer complexation .I. Preparation and characterization of a polyvinyl acetate phathalate-polyvinylpyrrolidone (PVAP-PVP) complex. Int. J. Pharm., 188: 221-232, 1999.
Takayama, K, and Nagai, T., Application of interpolymer complexation of polyvinylpyrrolidone/carboxyvinyl polymer to control of drug release. Chem. Pharm. Bull. 35: 4921-4927, 1987.
Zhang, L.M., Synergistic blends from aqueous solutions of two cellulose derivatives. Colloid Polym. Sci. 277: 886-890, 1999.
Rodriguez, F., Some general properties of polymer systems, Principles of polymer systems. McGraw-Hill, New York, 1970.
Jones, S. D., Woolfson, A. D., and Brown, A. F., Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymer. Int. J. Pharm., 151: 223-233, 1997.
Tan Y.T.F., Peh, K.K., and Al-Hanbali, O., Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech, 1 (3) article 24, 2000.
Corresponding Author: Yvonne Tze Fung Tan, School of Pharmaceutical Sciences, University of Science Malaysia, Minden 11800, Penang, Malaysia, Yvonne@usm.my
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps
/Y.Tan/Figure1.gif)
/Y.Tan/Figure2.gif)
/Y.Tan/Figure3.gif)
/Y.Tan/Figure4.gif)
/Y.Tan/Figure5.gif)
/Y.Tan/Figure6.gif)
/Y.Tan/Figure7.gif)
/Y.Tan/Figure8.gif)