J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(3):312-316, 2000
High Performance Liquid Chromatographic Determination of Cyclooxygenase II Inhibitor Rofecoxib in Rat and Human Plasma
Saeed Sattari and Fakhreddin Jamali1
Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, CanadaReceived November 3rd, 2000, Revised December 31st, 2000, Accepted December 31st, 2000
PDF version for printing
ABSTRACT
Rofecoxib is a relatively new non-steroidal anti-inflammatory drug with high selectivity in cyclooxygenase 2 inhibitory activity. There is only one assay reported for determination of the drug in biological samples. The assay requires a post-column UV reactor for photocyclization before detection with fluorescence detector. In addition, the internal standard (IS) used in the assay in not commercially available. We developed a new assay for determination of rofecoxib. Rat blank plasma (200 mL) or human blank plasma (500 mL) was spiked with rofecoxib to make final concentrations of 10 to 3000 ng/mL, and 100 ml of a 2 mg/mL of ketoprofen as IS, 100 ml of a pH 4.5 acetate buffer, and 6 mL of ethyl acetate were added. The resultant was vortex-mixed for 90 seconds and centrifuged at 2500 g for 3 min. The organic layer was separated and evaporated to dryness under vacuum. The residues were reconstituted in 170 mL of mobile phase and 150 mL was injected into an HPLC consisting of an autoinjector, an isocratic pump, a 10 cm × 4.6 i.d. C18 analytical column packed with 5 mm reversed phase particles, a variable UV spectrophotometer detector set at 272 nm, and an integrator. The mobile phase consisted of water (77%), acetonitrile (23%), acetic acid (0.1%), and triethylamine (0.03%) and was pumped at 1 mL/min at ambient temperature. The drug and IS were eluted at 13 and 24 min, respectively. The peak drug/IS area ratio versus drug concentrations relationship was linear (r>0.99). The extraction efficiency was >87%. The minimum quantifiable concentration was set at 10 ng/mL (correlation coefficient of <10%). This convenient, sensitive, and simple method is suitable to pharmacokinetic studies of rofecoxib in rats and humans.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are used in the treatment of various inflammatory diseases and pain (1). NSAIDs produce their pharmacological and toxic effect by inhibiting cyclooxygenases (COX)-2 and -1, respectively (2). Recently introduced NSAIDs, celecoxib and rofecoxib, selectively inhibit COX-2 and hence cause little or no gastrointestinal side effect (3). Thus far, only one assay has been reported for quantitation of rofecoxib (Fig.1) in plasma.
Figure 1: Chemical structure of rofecoxib
This method requires a fluorescence detector and post-column UV photocyclization of the parent drug and internal standard (4). In addition, the internal standard used in the assay is not commercially available. The objective of this work was to develop and validate an efficient, sensitive, and simple HPLC method for determination of rofecoxib in rat and human plasma using liquid-liquid extraction.
Experimental
Materials
Racemic ketoprofen was used as internal standard (IS) (Upjohn Company, Don Mills, Ontario, Canada). Ethyl acetate (Assurance grade) was obtained from BDH Chemicals Canada (Edmonton, AB, Canada). Acetonitrile (HPLC grade), Triethylamine, acetic acid and sodium acetate were all analytical grade and were purchased from Fisher Scientific (Edmonton, AB, Canada).
HPLC System
The instrument consisted of a Sil-9A model autoinjector, a SPD-6A model variable UV spectrophotometer detector set at 272 nm and a CR601 model Chromatopac integrator (Shimadzu, Japan). The autosampler window was blocked using aluminum foil to minimize the possibility of loss of drug potency due to the light sensitivity of the drug. The mobile phase, water-acetonitrile-acetic acid-triethylamine (77:23:0.1:0.03), was pumped through the system using a Waters 6000 A model HPLC pump (Waters, Mississauga, Canada) at a flow rate of 1mL/min. A 10 cm x 4.6 mm i.d. C18 analytical column packed with 5-mm reversed-phase particles (Chromatography Sciences Company, Canada) and a HPLC pre-column insert packed with C8 (Waters, MA, USA) were used for separation.
Standard solutions
Rofecoxib was extracted from Vioxx™ 25 mg tablets (Merck Frost, Kirkland, Quebec, Canada, Lot Eo13590). Twenty Vioxx tablets crushed to fine powder and suspended in 50 ml of HPLC grade ethyl acetate then shacked for 5 min and filtered. The residue was crystallized from acetonitrile after evaporation of the solvent in vacuum. During the extraction process, the containers were protected from direct light. A capillary melting point apparatus (Thomas Company, PA., USA) was used to determine the melting point. A differential scanning calorimetric (DSC 120, Seiko Instruments USA Inc. PA., USA) was utilized to record the thermal behavior of the powder. Nuclear magnetic resonance spectrum (1H NMR, 300 MHz, CDCl3) was recorded on a Bruker AM 300 (Bucker, Germany) retrofitted with a Tecmag MacSpect3 (Tecmag, TX, USA). A VG QUATTRO electrospray mass spectrometer at positive mode (Fisons Instruments, U.K) was used to record the mass spectrum. The extracted powder melted at 207-208C°. The differential scanning calorimetric spectrum also showed a sharp peak of heat absorption at 208.4 C°. The mass spectrum showed the correct fragmentation of the rofecoxib molecule (M+H at 315 and major fragments at 297, 269, and 237 of mass unit). In addition, the NMR spectrum also confirmed the structure to be rofecoxib.
A stock solution (10 mg/mL) of rofecoxib was prepared in ethyl acetate and kept in a lightproof container and refrigerated in order to reduce its degradation and photocyclization (4). Under these circumstances, the solution was stable at least for two months. Solutions of 1.0 and 0.1 mg/mL were prepared by diluting the stock solution in ethyl acetate before analysis. A 2 mg/mL ketoprofen (IS) stock solution was prepared by dissolving 2 mg of racemate powder in 10 mL of methanol and sufficient 0.01 M NaOH to 100 mL. Standards curves were prepared by adding 100 mL of ketoprofen and various volumes of rofecoxib stocks solutions to 200 mL of rat or 500 mL of human blank plasma to make the final concentrations of 10, 25, 50, 100, 250, 500, 1000, 2000, 3000 ng/mL.
Plasma extraction procedure
To the test tubes containing 200 mL of rat or 500 mL human plasma were added 100 mL of 2 mg/mL IS solution, 100 mL of pH 4.5 acetate buffer (0.05 M) and 6 mL ethyl acetate. The tubes were vortex-mixed for 90 seconds and centrifuged at 2500 g for 3 min. The organic layer was transferred to a clean tube and evaporated to dryness (Savant Speed Vac concentrator-evaporator, Emerson Instruments, Scarborough, Canada). The residue was dissolved in 170 mL of mobile phase and an aliquot of 150 mL was injected into the HPLC.
Stability
Rofecoxib stock solutions were protected from direct light by rapping aluminum foil around its containers and keeping the solution at +4° or at room temperature. The stability of these solutions was compared against a freshly prepared stock solution and one that was kept at the room temperature.
Extraction efficiency
Various organic solvents such as chloroform, dichloromethane and hexane alone or in combinations were tested. Ethyl acetate was chosen due to its greater extraction efficiency. 200 mL of blank rat plasma was spiked with rofecoxib stock solution to make the final concentrations of 100, 500 and 1000 ng/mL (n=3). These samples were extracted and evaporated as mentioned before. The residues were dissolved in 1 mL mobile phase and 150 mL was injected into HPLC. The peak area of rofecoxib from the spiked plasma samples were compared with the peak areas obtained after direct injection of 150 mL of 100, 500, and 1000 ng/mL rofecoxib solutions.
Accuracy and precision
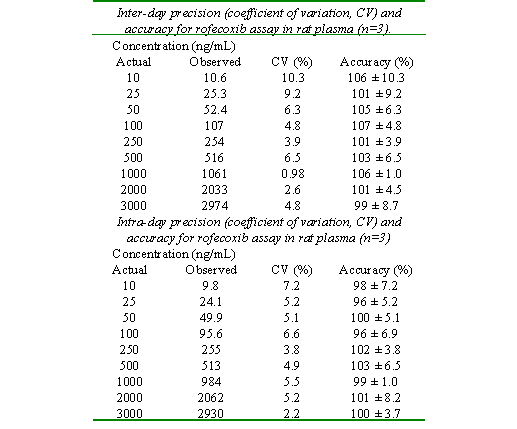
Aliquots of 200 mL of blank rat plasma were spiked with 100 mL of 2 mg/mL of IS and of rofecoxib solutions with various strengths to yield concentrations of 10, 25, 50, 100, 250, 500, 1000, 2000, and 3000 ng/mL. Each sample was prepared in triplicate on three consecutive days. Accuracy was expressed as the mean % error, [(mean measured concentration )/(expected concentration)] × 100. Precision was calculated as inter and intra-day coefficient of variation [%CV=(SD/mean) × 100]. Least-squared regression method was used to determine the regression coefficients and the equation for the best fitting line.
Rofecoxib determination in rat and human plasma
Solutions of 2.5 and 5 mg/mL of rofecoxib were prepared in polyethylene glycol 400 for i.v. and oral dosing. Four male Sprague Dawley rats (300 ± 20 g) were cannulated (5). After overnight recovery, two rats were received 2.5 mg/kg i.v. bolus and two others were administered 5 mg/kg oral doses through gastric gavage. Blood samples were taken at 0, 5, 10, 20, 30, 45 min and 1, 1.5, 2, 3, 4, 6 and 8 h through the jugular catheter. Plasma was separated by centrifugation for 2 min at 10000 g and kept at -20º until analyzed.
In an attempt to investigate the applicability of the assay in humans, a 25 mg Vioxx tablet (Merck-Frosst, Kirkland, PQ, Canada) was orally administered to a 75 kg, 42 year-old healthy male volunteer. Written informed consent was obtained before administering the tablet. Venous blood samples were collected at 0, 3 and 24 h post-dose. Plasma was harvested and kept at -20º until analyzed.
Treatment of Data
All pharmacokinetic indices were estimated using WinNonlin version 3.0 (Pharsight Corporation, California, USA). The non-compartmental model contained in the program was used.
Results and Discussion
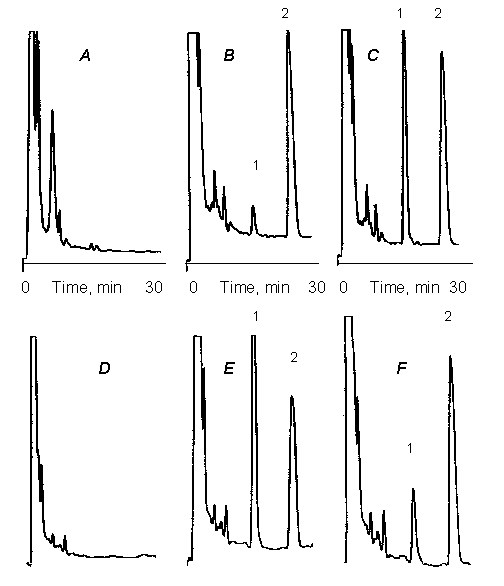
Rofecoxib and IS appeared on the chromatograph in approximately 13 and 24 min respectively with no interfering peaks (Fig. 2). The run time of the assay, therefore, was set at 30 min.
Figure 2: Representative 30 min chromatographs depicting rofecoxib (peak 1) and internal standard (peak 2). A) blank rat plasma: B) rat plasma spiked with 15 ng/mL of rofecoxib; C) rat plasma sample 6 h after oral administration of 5 mg/kg; D) blank human plasma, E) human plasma 3 h; F) human plasma 24 hr after a single 25 mg oral dose of rofecoxib tablet (Vioxx)
Excellent linearity observed between the peak area ratios and drug concentrations over the range of 10 to 3000 ng/mL (r>0.99). The minimum quantifiable concentration was set at 10 ng/mL based on 200 mL of the rat and 500 mL of human plasma. However, in 500 mL human plasma, a concentration of 4 ng/mL of the drug was measurable with intra-day CV of <10%.
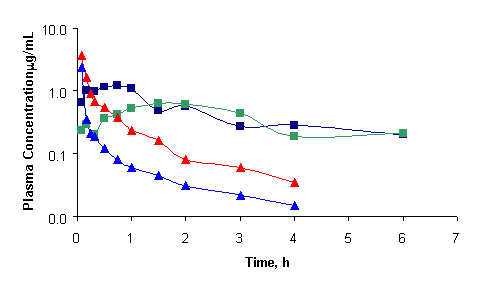
The accuracy of the assay was >90% and CV did not exceed 10% (Table 1). The sensitivity of the assay is sufficient for determination of rofecoxib at least 6 h and 24 h after administration of single oral doses of 5 mg/kg (Fig. 3) and 25 mg to the rat and humans, respectively.
Figure 3: Individual plasma concentration -time profile of rofecoxib after administration of 2.5 mg/kg bolus i.v. (
) and 5 mg/kg oral doses (
) to four rats. Lines join experimental data points.
Table 1: Assay Validation Data
The stock solution of rofecoxib was stable at least for two month when it was protected from direct light by rapping aluminum foil around its container and kept refrigerated at +4° or at room temperature. Direct exposure of rofecoxib solution to the laboratory light resulted in degradation of the drug and appearance of a major HPLC peak 3 min before the drug. Rofecoxib crystals extracted from tablets maintained their potency at least for a 4 months period of storage in a container rapped in aluminum foil and kept refrigerated.
Based on the limited data (n=2/group), in the rat, rofecoxib exhibited a multi-compartment disposition kinetics after i.v. doses. Assuming linear pharmacokinetics, the average absolute bioavailability was 60% following oral administration. After 5 mg/kg oral doses, peak concentrations of 640 and 1200 ng/mL were observed in the two rats examined. The drug had a terminal t1/2 of approximately 2 h (2.1 and 2.4 h after oral; 1.7 and 1.9 h after i.v. administration) and a volume of distribution of 5.2 to 7.2 L/kg. The systemic and oral clearance of the drug was 36-110 and 29-34 mL/min/kg, respectively.
We cannot offer a reasonable explanation for the observed variability due to the limited sample size used. The only other data available from animals are after administration and determination of [14C] rofecoxib (6). The authors reported the half-life of 6.7 and 2.6 h in rats and dogs, respectively.
Following oral administration of a 25 mg dose of rofecoxib to a human subject, plasma concentrations of 330 and 47 ng/mL were observed at 3 and 24 h post-dose, respectively (Fig. 2).
Conclusion
The HPLC assay presented here is suitable for determination of rofecoxib in the rat and human plasma following therapeutic doses. This assay is simple and sensitive and there is no need of utilizing the fluorescence detector and post-column UV photoreactor.
Acknowledgements
This work was financially supported by grant number G118160210 of Canadian Institute of Health Research and Canadian Arthritis Network (Networks of Centres of Excellence).
References
- Cappel, M. S., Schein, J. R. (2000) Diagnosis and treatment of nonsteroidal anti-inflammatory drug-associated upper gastrointestinal toxicity. Gastroenterol. Clin. North Am. 29: 97-124.
- Cryer, B., Feldman, M. (1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am. J. Med. 104: 413-421.
- Bolten, W. W. (1998) Scientific rationale for specific inhibition of COX-2 J. Rheumatol. 25:Suppl. 51, 2-7.
- Woolf, E., Fu I., Matuszewski, B. (1999) Determination of rofecoxib, a cyclooxygenase-2 specific inhibitor, in human plasma using high-performance liquid chromatography with post-column photochemical derivatization and fluorescence detection. J. Chromatogr. B, 730: 221-227.
- Kulmatychi KM and Jamali F. (1997) pharmacodynamics of sotalol enantiomers are influenced by inflammation with no pharmacokinetics consequence. Pharm Res 14: S511.
- Rita, A., Halpin, L. A., Geer, K. E., Zhang, T. M., Marks, D. C., Dean, A. N., Jones, D. M., George, D., and Kamlesh, P. v. (2000) The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective Cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metaol Dispo 28: 1244-1254.
Corresponding Author: Dr. F. Jamali, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Alberta, Canada, T6G 2N8, fjamali@pharmacy.ualberta.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps