J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(1):125-136, 2000
Modulation of the Pharmacokinetics and Pharmacodynamics of Proteins by Polyethylene Glycol Conjugation
Manuscript received February 25, 2000, Revised April 17th, 2000; Accepted April 18th, 2000.
Reza
Mehvar1,
School
of Pharmacy, Texas Tech University Health Sciences Center, 1300 Coulter,
Amarillo, Texas, USA
PDF version for printing
Introduction
With the rapid advances in the field of biotechnology during the last decade, many peptides and proteins have been produced and evaluated for therapy of various diseases, including cancer. However, rapid clearance and the possibility of immunogenicity after the in vivo administration of these biotechnology-driven products have impeded their marketing. To circumvent these problems, synthetic and natural polymers such as polyethylene glycol (PEG) and dextrans, respectively, have been covalently attached to proteins, and some of these protein-polymer conjugates have shown promising therapeutic results. The conjugation of proteins with polymers usually causes a reduction in the recognition of the protein by the immune system, resulting in a decrease in protein clearance and immunogenicity. Most of the protein-polymer conjugates retain the pharmacologic activity of the protein, although to a lesser extent than the native protein. Additionally, in most of the examples in the literature, a significant increase in the plasma half life of the protein more than compensates for any reduction in the pharmacologic effects of the polymer-protein conjugates. Therefore, polymer conjugation in most cases would result in a net increase in the pharmacologic activity of the protein.
The intent of this article is to review the pharmacokinetics and pharmacodynamics of proteins conjugated to PEG which is one of the most widely used synthetic polymers for protein conjugation.
Physiochemical Properties
Polyethylene glycol (PEG) is a polymer with the structure (–CH2CH2O–)n that is synthesized normally by ring opening polymerization of ethylene oxide. The polymer is usually linear at molecular weights (MWs) £ 10 kD. However, the higher MW PEGs may have some degree of branching. Polyethylene glycols of different MWs have already been used in pharmaceutical products for different reasons (e.g., increase in solubility of drugs). Therefore, from the regulatory standpoint, they are very attractive for further development as drug or protein carriers.
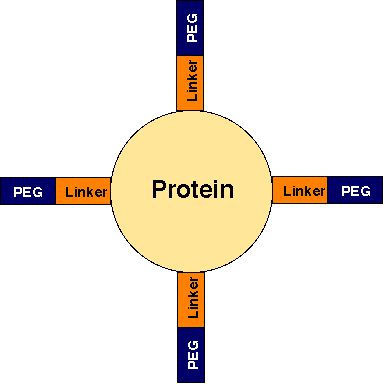
For coupling proteins to PEG, usually monomethoxy PEG [CH3 (–O–CH2–CH2)n–OH] is first activated by means of cyanuric chloride, 1,1'-carbonyldiimidazole, phenylchloroformate, or succidinimidyl active ester (1) before the addition of the protein. In most cases, the activating agent acts as a linker between PEG and the protein, and several PEG molecules may be attached to one molecule of protein as depicted in Figure 1. Therefore, pharmacokinetics and pharmacodynamics of the PEG-protein conjugates are dependent on the MW of the PEG used for conjugation, the number of PEG molecules per each molecule of protein, and the nature of the bond between the protein and the linker. Interested readers are referred to a comprehensive review of the PEG-protein coupling methods by Deluged et al. (1).
Figure 1. Schematic presentation of a protein-PEG conjugate. The number of PEG molecules per each protein molecule varies for different conjugates.
In Vivo Disposition of PEG Backbone
It is believed that the kinetics of proteins attached to polymers are substantially affected by the kinetics of the polymer itself. Therefore, before reviewing specific PEG-protein conjugates, an analysis of the plasma kinetics and tissue distribution of PEGs is necessary.
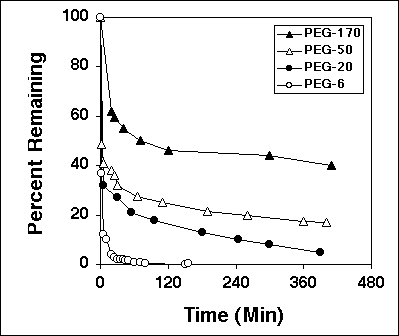
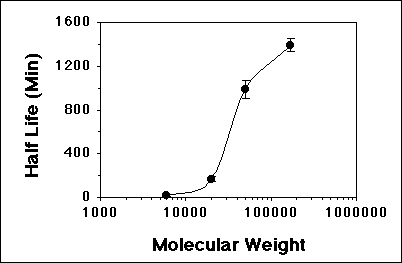
The plasma kinetics of PEGs are reported (2, 3) to be dependent on both the MW of the polymer and the site of injection. Yamaoka et al. (2) investigated the disposition of radiolabeled PEGs with MWs of 6 kD (PEG-6), 20 kD (PEG-20), 50 kD (PEG-50), and 170 kD (PEG-170) after iv administration to mice. Similar to other polymers such as dextrans (4, 5), the plasma concentrations (Fig. 2) and area under the plasma concentration-time curves (AUCs) (Table 1) of higher MW PEGs were substantially greater than those of the lower MW polymers. Additionally, the half life of the polymers progressively increased as the MW increased from 6 kD to 170 kD (Table 1); the relationship between the half and the MW of PEGs is sigmoidal (Figure 3), which appears to be one of the characteristics of the kinetics of macromolecules.
Figure 2. Blood radioactivity-time courses after iv administration of PEG with different molecular weights. Key: (s) PEG-170; (∆) PEG-50; (l) PEG-20; (m) PEG-6. From Ref. (2).
Table 1. Mean ± SD of AUC and terminal half life of PEGs with different MWs after iv administration to micea
|
Parameter |
PEG-6 |
PEG-20 |
PEG-50 |
PEG-170 |
|
AUC, |
6.17 |
110 |
600 |
1110 |
|
t1/2, min |
17.6 |
169 |
987 |
1390 |
a Source:
Reference (2)
With regard to the site of injection, PEG-50 is retained at the injection site longer than PEG-6 after im and sc injections (3), suggesting that the absorption of PEG from im and sc sites is MW dependent. However, after the ip administration, the injection site disappearance profiles of both MWs were very similar (3).
Figure 3. Relationship between the plasma half life of PEG and its molecular weight. From data presented in Table 1, Ref. (2).
The differences among the plasma concentration-time courses of PEGs with different MWs (Fig. 2) are mostly due to the size of these PEGs in relation to the pore sizes of the vascular beds in kidneys. Chang et al. (6) reported that, in rats, renal elimination of another linear polymer, neutral dextrans, with a MW of ~ 10 kD occurred without any molecular restriction. However, the renal clearance of dextrans of larger MWs progressively decreased and approached zero at a MW of ~40 kD. This is in agreement with a study (2) in mice using radiolabeled PEG, demonstrating a sigmoidal relationship between the renal clearance and the log MW of PEGs. This type of sigmoidal relationship (2) agrees well with the theoretical models of renal excretion of macromolecules based on the pore sizes of the glomerular capillary wall.
The relatively limited information on the metabolism of PEG in the body (7, 8) indicates that PEG undergoes cytochrome P-450 oxidation, resulting in the formation of ketone, ester, and aldehyde groups (8). Additionally, smaller MW PEGs are excreted into bile (7).
In terms of tissue distribution, it appears that PEGs with MWs between 6 kD to 170 kD distribute insignificantly to tissues such as heart, lung, liver, spleen, kidney, and thyroid gland (2). However, the distribution of PEGs to gastrointestinal tract and feces is relatively substantial (2). Additionally, no clear MW dependency is observed for the accumulation of PEG in tissues (2).
PEG-Protein Conjugates
During the last three decades, PEG has been investigated extensively for delivery of various proteins via parenteral routes. Some examples are listed below.
Anticancer
Agents
Generally, polymers have been most widely used for the delivery of both traditional (small molecule) drugs and proteins/enzymes in the treatment of cancer. However, PEGs have been specifically investigated for the delivery of anticancer proteins/enzymes as discussed below.
Antibodies: One of the major problems for the use of xenogenic monoclonal and polyclonal antibodies for the treatment of tumors is their immunogenicity which results in a rapid removal of the antibodies from the body and the possibility of allergic reactions after multiple administration. Kitamura et al. (9) conjugated the F(ab')2 fraction of the murine monoclonal antibody A7 to PEG 5 kD and studied the tumor accumulation and the kinetics of the conjugate in mice. The conjugate had a longer plasma half life and higher tumor accumulation, compared with the free F(ab')2 fraction. However, the tumor: blood ratio of the free F(ab')2 fraction was higher than that for the conjugate (9).
Takashina et al. (10) studied the pharmacokinetics and dynamics of conjugates of monoclonal antibody A7 to PEG 5 kD and dextran 70 kD. In vitro studies showed that the conjugates retained the antigen binding activity of the antibody. Additionally, after the iv administration of the conjugated and free antibody, the PEG conjugate had a plasma half life twice of that for the free antibody (10). On the other hand, the dextran conjugate showed higher clearance and shorter half life, compared with the free and PEG conjugated antibody. Additionally, the tumor accumulation of dextran-antibody conjugate was less than those for the free and PEG conjugated antibody. This study (10) suggests that the kinetics of polymer-monoclonal antibody A7 are significantly dependent on the structure of the polymer.
Arginase: A PEG 5 kD conjugate of arginase retained 65% of the activity of the enzyme and prolonged its plasma half life in mice after multiple dose therapy (11); 30 days after the start of the treatment, the half life of the native enzyme was 1 hr, while the half life of the conjugate was 12 hr. In terms of effects, the conjugate increased the survival time in mice with Taper liver tumor. However, the free enzyme did not show any improvement in the survival time (12). With regard to the effects of the enzyme against L5178Y mouse leukemia cells, whereas the conjugate was more effective than the native enzyme in vitro, neither was able to stop the growth of tumor in vivo (12).
Asparaginase: Asparaginase, isolated from Escherichia Coli and Erwinia Carotovora, metabolizes asparagine, a necessary nutrient for sensitive tumors. However, after multiple injection of the enzyme, antibodies raised against the enzyme would quickly remove the enzyme from the circulation, and also significant immunogenicity may be observed. Several studies (13-19) have documented the usefulness of a conjugate of asparaginase with PEG for the treatment of various cancers in both humans and animals. Ho and his colleagues (15, 17) showed that the conjugate would alter the pharmacokinetics of the enzyme drastically in both humans and rabbits. In humans (15), conjugation resulted in an increase in the plasma half life from 20 hr (for native enzyme) to 357 hr (for the conjugate). In rabbits (17), the half life values of the free and conjugated asparaginase were 20 and 144 hr, respectively. The increase in the plasma half lives in both species was due to a significant decrease in the clearance of the enzyme (15, 17). The alterations in the kinetics of the enzyme by PEG conjugation also resulted in significant improvements in the toxicity and efficacy profile of the enzyme after in vivo administrations to animals (14, 16, 18) and humans (13, 19). A conjugate of asparaginase and PEG (pegaspargase) was marketed (OncasparÒ) in 1994 for the treatment of acute lymphoblastic leukemia (ALL) in patients who are hypersensitive to native forms of L-asparaginase. OncasparÒ is marketed by Rhône-Poulenc Rorer Pharmaceuticals, Inc. in the U.S. and Canada.
Methioninase: It is known that all the tumor cells have elevated requirement for methionine. Therefore, methioninase may be used in cancer therapy. However, the recombinant enzyme, obtained from bacteria, has a short plasma half life and may be immunogenic upon multiple dose administration. Very recently, Tan et al. (20) demonstrated the potential of a conjugate of methioninase and PEG 5 kD in cancer therapy. In vitro tests demonstrated that the conjugate retained 70% activity of the enzyme. Additionally, in rats, the plasma half life of the enzyme was increased by a factor of 2 when it was conjugated to PEG 5 kD (20). Further, the effects of the conjugate lasted for 8 hr, as opposed to 2 hr for the free enzyme. In vitro studies in human lung and kidney cancer cells showed identical IC50 values for the conjugated and free methioninase, demonstrating the effectiveness of the enzyme in the conjugated from. Also, after the injection of the conjugate to tumor-bearing mice, the tumor: blood enzyme ratio was higher for the conjugate (1:6), compared with the free enzyme (1:10) administration (20). More studies are needed to confirm these promising findings.
Enzyme
Replacement
Adenosine Deaminase: A deficiency of the enzyme adenosine deaminase (ADA) results in combined immunodeficiency disease (CID). For several years, conjugates of PEG and ADA have been used successfully for enzyme replacement in the treatment of CID in children (21-23). A conjugate of PEG and ADA, which is also named pegademase, was marketed (AdagenÒ) by Enzon, Inc. (Piscataway, NJ) in the US in 1990. The outcome of therapy with the conjugate appears to be better than red blood cell transfusion (23), which is another treatment for ADA deficiency. Studies (21-23) have shown that weekly intramuscular injections of the conjugate of PEG with bovine ADA would reverse the symptoms of ADA deficiency in most cases without substantial toxicity or hypersensitivity. The conjugate appears to have a very long half life of 48-72 hr in children (21). From a historic perspective, the PEG-ADA conjugate served as one of the earliest examples of polymer conjugates marketed in the US and prompted more research interest in this area.
Uricase: Uricase is an enzyme which converts uric acid to allantoin and is lacking in humans. When the enzyme is administered to humans, it causes a significant reduction in the plasma and urine levels of uric acid. Therefore, it can be effective in the treatment of gout and other diseases related to high levels of urate. However, after multiple administration, the antibodies against this enzyme would deactivate it very rapidly. Several conjugates of uricase with PEGs (24-28) have been investigated to overcome this problem. Yasuda et al. (28) reported that conjugation of uricase with PEG resulted in a decrease in antibody production and reactivity towards uricase. When administered intravenously to rats, the enzymatic activity half life of the PEG conjugate (~ 7 hr) was almost 10 times of that for the parent enzyme (0.6 hr) (28).
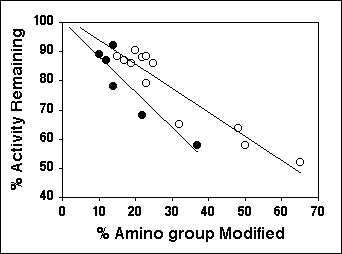
Figure 4. Relationship between the percentage of amino groups of uricase modified with dextran 10 kD (m) or PEG 10 kD (l) and the percentage of remaining enzymatic activity of uricase. From Ref. (28).
The enzymatic activity of the polymer conjugated uricase is shown to be dependent on the degree of modification of the amino groups of the enzyme during the conjugation process (28). An increase in the modification would result in a decrease in the enzymatic activity of uricase for both dextran and PEG conjugates (Fig. 4). However, the decrease in the activity is more pronounced for the PEG conjugate, compared with dextran conjugation (Figure 4) (28).
Antioxidant
Enzymes
Catalase: Similar
to superoxide dismutase (SOD), catalse is an antioxidant enzyme, and
several studies (29-32) have investigated the effects of PEG conjugates
of SOD and catalse on the same animal model. The PEG-catalse conjugate
was first prepared by Abuchowski et al. (33) using both PEG 1900 and 5
kD. These investigators (33) demonstrated that both conjugates retained
significant (>90%) activity of the enzyme and were resistant to
digestion by trypsin, chymotrypsin, and protease. Further, the half life
of the conjugates was long even after their repeated administration to
mice (33). A later study (34) using subcutaneous osmotic pumps
delivering the conjugate showed the conjugate’s beneficial effects in
a rat model of lung injury due to asbestosis. Despite promising effects
of the PEG-catalase conjugate, recent work in this area has concentrated
more on a conjugate of SOD and PEG described below.
Superoxide Dismutase (SOD): Among the conjugates of PEG, SOD is the most widely studied. Superoxide dismutase is an antioxidant enzyme which eliminates superoxide anion, reducing tissue injury. After its iv administration in animals, the plasma half life of the enzyme is very short (5-10 min). Several investigators have reported the effects of conjugation of SOD with PEG on the pharmacokinetics and dynamics of the enzyme, some of which are summarized in Table 2 (29-32, 35-47). Although some of these studies have compared the effects of PEG-SOD with those of the free enzyme, most of the studies have concentrated on the effects of PEG-SOD without a comparison with the free SOD (Table 2). There is little doubt that conjugation of SOD with PEG increases its plasma half life (35) and reduces its immunogenicity (29, 35). However, conflicting reports (30-32, 36-40, 43, 45-47) exist with regard to the effects of PEG-SOD in various animal models of injury. Additionally, the results of clinical trials (42, 44) with PEG-SOD have not been unequivocal.
Table 2. Some of studies on the conjugates of PEG and SOD.
|
Type of
Study |
Comments |
Reference |
|
In vitro and
in vivo kinetics and dynamics in rats |
PEG 5 kD
conjugate retained 51% enzyme activity; plasma half life of
conjugate was longer than the native SOD after repeated dosing;
anti-inflammatory effect of the conjugate was higher than SOD. |
(35) |
|
In vivo
immunogenicity in mice |
Decreased
immunogenicity; antibody titer to the conjugate was 0.03%-0.07%
of that observed with SOD. |
(29) |
|
In vivo
effects in endotoxemia in pigs |
No beneficial
effects |
(30) |
|
In vivo
effects in a dog model of ischemia/ reperfusion |
Conflicting
results: both no effect (37) or a reduction (36) in heart injury
associated with reperfusion have been reported. |
(36, 37) |
|
In vivo
effects in a rat model of brain ischemia |
Administration
of PEG-SOD before induction of focal cerebral ischemia resulted
in a reduction in brain injury. |
(31) |
|
In vivo
effects in a rabbit model of ischemia/ reperfusion |
No effect in
heart injury associated with reperfusion. |
(38) |
|
In vivo
distribution into brain of piglets |
IV injection
of PEG-SOD did not increase the enzyme level in the brain in
control piglets and in animals subjected to global
ischemia/reperfusion. |
(39) |
|
In vivo
effects in hemorrhagic shock in rats |
Administration
of a PEG-SOD conjugate to a rat model increased survival from
25% to 67%. |
(40) |
|
In vivo brain
distribution in rats |
The
concentrations of PEG-SOD in the brain and CSF of normal rats
were low; brain and CSF concentrations were higher after
hypertensive brain injury |
(41) |
|
In vivo
effects in piglets with hypoxic brain injury |
Administration
of PEG-SOD 5 min after reoxygenation did not have any positive
effects. |
(32) |
|
Phase II
clinical trial study in severe head injury |
Improved
outcome at 3 and 6 months after the treatment with PEG-SOD
(10,000 U/kg), compared with placebo. |
(42) |
|
In vivo study
in rats with oxygen toxicity |
Insufflation
of PEG-SOD increased survival time, in comparison with both
placebo and free SOD. |
(43) |
|
Clinical trial
in severe head injury |
Percent of
patients in a vegetative state or dead at 3 and 6 months
postinjury was lower after the conjugate, compared with placebo. |
(44) |
|
In vivo effect
in a rat model of ischemic renal failure |
The PEG-SOD
was more effective than an equivalent dose of free SOD. |
(45) |
|
In vivo
effects in a rat model of warm renal ischemia |
PEG-SOD
conjugates were more protective, compared with free SOD. |
(46) |
|
In vivo
effects in a rat model of ischemia/reperfusion |
SOD conjugated
to PEG showed a superior effect over that conjugated to
polyacryloylmorpholine. |
(47) |
|
|
|
|
Thrombolytic Agents
Streptokinase: Rajagopalan et al. (48) conjugated streptokinase to PEG 2 kD, 4 kD, and 5 kD, and investigated the thrombolytic activity and antigenicity of the conjugates. In vitro studies demonstrated comparable activity for the conjugates and the free enzyme. However, the binding of the conjugates to antibodies against streptokinase was reduced by 95% (48). In vivo studies in mice (48, 49) revealed low clearance of the conjugates attached to plasmin, resulting in a half life of 200 min for the conjugate, compared to a half life of 15 min for streptokinase itself. These studies (48, 49) demonstrate that PEG conjugation of streptokinase retains the activity of the enzyme, prolongs its plasma circulation by blocking plasmin degradation, and reduces the antigenicity of the enzyme.
Urokinase: In dogs, a conjugate of urokinase, a thrombolytic agent, with PEG 5 kD was shown (50) to have longer activity and more activation of fibrinolysis, compared with the native enzyme. Also, a polypropylene glycol-PEG conjugate of urokinase showed a decreased activity on plasminogen and had a longer plasma half life in rabbits, compared with the native enzyme (51). Later (52), it was shown that this conjugate blocked autolysis of the enzyme at 370C. Unfortunately, these early positive results have not been followed by more extensive in vivo studies.
Oxygen Carriers
Hemoglobin: Several studies have examined the feasibility of the conjugation of hemoglobin to PEG for use as a blood substitute. Hemoglobin binds to oxygen and can be used as an oxygen carrier. However, because of its rapid elimination, the plasma half life of the protein is very short. Additionally, the affinity of hemoglobin to oxygen is too high for release of oxygen in the tissues. A conjugate of PEG with pyridoxylated hemoglobin has been shown (53, 54) to have longer plasma half life and better therapeutic effects in rats, compared with the free hemoglobin. The benefits of PEG-hemoglobin conjugates as a blood substitute have been shown in several animal models, including a hemorrhagic hypotension pig model (55) and in partial exchange transfusion and top-loaded rat models (56). Additionally, a PEG-hemoglobin conjugate has been used (57) for an increase in the sensitivity of tumors to radiation by increasing oxygen delivery to the tumor. These studies point to the potential of hemoglobin conjugated to PEG for manipulation of the oxygen levels in normal and malignant tissues.
Cytokines
and Hematopoietic Growth Factors
Interleukin-2 (IL-2): Both animal and clinical studies have been conducted using PEG conjugates of IL-2. Earlier studies in animals (58, 59) and humans (60) showed that PEG conjugation would increase stability, decrease clearance, and increase plasma half life (> 20 fold) of IL-2. Further, these studies (58-61) suggested promising effects for the PEG-IL-2 conjugate in the treatment of various cancers. However, more recent data (62-64), mostly in patients, have failed to clearly demonstrate an advantage for PEG-IL-2, compared with free IL-2, in terms of therapeutic or toxic end points for the treatment of cancer. On the other hand, it appears that recent interest in the PEG-IL-2 conjugate revolves around its potential beneficial effects in patients with human immunodeficiency virus (65-69). Recent studies (65-69) in patients with HIV show that low dose PEG-IL-2, alone or in combination with zidovudine, would increase the immune response by increasing the number of CD4 T cells without significant toxicity. Additional clinical studies, comparing free and PEG conjugated IL-2 will shed more light on these exciting results.
Recombinant human granulocyte colony-stimulating factor (rhG-CSF): This is a 156 amino acid glycoprotein which is produced by Escherichia Coli and increases production and phagocytic and cytotoxic activities of neutrophils (70). The plasma half life of rhG-CSF is short (3.5 hr) (70), requiring daily injections to sustain the neutrophil levels in situations like cancer chemotherapy. In 1991, Tanaka et al. (71) reported that a conjugate of rhG-CSF with PEG increased the plasma half life of the growth factor from 1.8 hr (native factor) to 7 hr (conjugated factor) in mice. The increase in half life was associated with an increase in both the intensity and duration of the effect of the drug on the neutrophil count (71). These results were later (72) confirmed in mice made neutropenic by the administration of anticancer agents cyclophosphamide and fluorouracil. Recent studies (73-75) demonstrated that the in vivo activity of the conjugate is dependent on both the MW of PEG (73, 74) and the total number of PEG units attached to rhG-CSF (73, 75); there was a positive relationship between the total mass of the conjugate and the intensity and duration of the effect of rhG-CSF. Future studies should be conducted to determine whether these positive results in animals can be extended to humans.
Recombinant human granulocyte/macrophage colony-stimulating factor (rhGM-CSF): This is a 127 amino acid glycoprotein produced in yeast which acts similar to rhG-CSF to increase neutrophils, with a broader action on monocytes, macrophages, and eosinophils (70). Similar to rhG-CSF, the plasma half life of rhGM-CSF is short (2-3 hr) (70), requiring daily injections to sustain the neutrophil levels in patients undergoing bone marrow transplantation or intensive chemotherapy. Compared with rhG-CSF, the studies on the conjugates of PEG with rhGM-CSF are scarce (76, 77). The limited information indicates that similar to rhG-CSF, PEG conjugation increases the plasma half life (76) and some biological activities of rhGM-CSF (77).
Other
Proteins
Table 3 lists the use of PEGs for delivery of some other therapeutic agents (78-86) which are not discussed in detail in this review. These studies (Table 3) show that polymer conjugation could result in altered pharmacokinetics, decreased affinity of the conjugate to bind to the protein receptor, and/or a decrease in antigenicity of proteins.
Concluding Remarks
The examples provided in this review clearly point to the potential advantages of polyethylene glycols for parenteral delivery of proteins. Despite significant promise of protein therapeutics in cell culture and other in vitro studies, optimal delivery of these agents in humans is very challenging. This is mostly because of relatively high clearance and short plasma half life of these agents, especially after multiple administration which results in activation of the immune system and faster elimination of the proteins. The available studies on the use of PEG for delivery of proteins indicate that these polymers will continue to have a significant role in the delivery of proteins in the future.
Table 3. Additional studies on the conjugates of PEGs with proteins
|
Drug/Protein |
Description |
|
Antigen E |
A preliminary
study in man showed that a 5 kD conjugate may be useful for the
immunotherapy of ragweed hay fever (78). |
|
Batroxobin |
A 10 kD
conjugate retained the activity of the enzyme while losing its
ability to bind to anti-batroxobin antibodies in dogs (79). |
|
Bilirubin
oxidase |
In a rat model
of jaundice, the conjugate reduced the blood and liver levels of
bilirubin, but, did not improve the liver function tests (80). |
|
Honeybee Venom |
In a clinical
study, a 5.7 kD conjugate showed lower systemic reactions during
immunotherapy and less efficacy against honeybee sting (81). |
|
Interferon-alpha |
In humans, the
half life of the conjugate was twice as long as that of free
protein; however, this did not result in a substantial reduction
in the frequency of the protein administration (82). |
|
Interferon-gamma |
A 5 kD
conjugate had activity similar to that of free protein but with a
reduced binding affinity; the plasma half life of the conjugate
was significantly longer than that of free protein in rats (83). |
|
Interleukin-6 |
A 12 kD
conjugate showed significantly higher thrombopoietic effects
(increase in the platelet counts), compared with free IL-6 in mice
(84) |
|
Tissue
Plasminogen Activator |
In mice, the
half life of radioactivity after the injection of the radiolabeled
conjugate with 5 kD and 20 kD was long; however, the effect
disappeared much faster (85). |
|
Trypsin |
A 5 kD
conjugate was resistant to anti-trypsin antibody precipitation and
retained some of the activities of trypsin to varying degrees
(86). |
References
-
Delgado C; Francis GE; Fisher D. The uses and properties of PEG-linked proteins. Crit Rev Ther Drug Carrier Sys, 9:249-304, 1992.
-
Yamaoka T; Tabata Y; Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci, 83:601-606, 1994.
-
Yamaoka T; Tabata Y; Ikada Y. Fate of water-soluble polymers administered via different routes. J Pharm Sci, 84:349-354, 1995.
-
Mehvar R; Shepard TL. Molecular weight-dependent pharmacokinetics of fluorescein-labeled dextrans in rats. J Pharm Sci, 81:908-912, 1992.
-
Mehvar R; Robinson MA; Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci, 83:1495-1499, 1994.
-
Chang RLS; Ueki IF; Troy JL; Deen WM; Robertson CR; Brenner BM. Permselectivity of the glomerular capillary wall to macromolecules-Experimental studies in rats using neutral dextran. Biophys J, 15:887-906, 1975.
-
Friman S; Egestad B; Sjovall J; Svanvik J. Hepatic excretion and metabolism of polyethylene glycols and mannitol in the cat. J Hepatol, 17:48-55, 1993.
-
Beranova M; Wasserbauer R; Vancurova D; Stifter M; Ocenaskova J; Mara M. Effect of cytochrome P-450 inhibition and stimulation on intensity of polyethylene degradation in microsomal fraction of mouse and rat livers. Biomaterials, 11:521-4, 1990.
-
Kitamura K; Takahashi T; Takashina K; Yamaguchi T; Noguchi A; Tsurumi H; Toyokuni T; Hakomori S. Polyethylene glycol modification of the monoclonal antibody A7 enhances its tumor localization. Biochem Biophys Res Commun, 171:1387-94, 1990.
-
Takashina K; Kitamura K; Yamaguchi T; Noguchi A; Noguchi A; Tsurumi H; Takahashi T. Comparative pharmacokinetic properties of murine monoclonal antibody A7 modified with neocarzinostatin, dextran and polyethylene glycol. Jpn J Cancer Res, 82:1145-1150, 1991.
-
Savoca KV; Abuchowski A; van Es T; Davis FF; Palczuk NC. Preparation of a non-immunogenic arginase by the covalent attachment of polyethylene glycol. Biochim Biophys Acta, 578:47-53, 1979.
-
Savoca KV; Davis FF; van Es T; McCoy JR; Palczuk NC. Cancer therapy with chemically modified enzymes. II. The therapeutic effectiveness of arginase, and arginase modified by the covalent attachment of polyethylene glycol, on the taper liver tumor and the L5178Y murine leukemia. Cancer Biochem Biophys, 7:261-8, 1984.
-
Park YK; Abuchowski A; Davis S; Davis F. Pharmacology of Escherichia coli-L-asparaginase polyethylene glycol adduct. Anticancer Res, 1:373-6, 1981.
-
Abuchowski A; Kazo GM; Verhoest CR, Jr.; Van Es T; Kafkewitz D; Nucci ML; Viau AT; Davis FF. Cancer therapy with chemically modified enzymes. I. Antitumor properties of polyethylene glycol-asparaginase conjugates. Cancer Biochem Biophys, 7:175-86, 1984.
-
Ho DH; Brown NS; Yen A; Holmes R; Keating M; Abuchowski A; Newman RA; Krakoff IH. Clinical pharmacology of polyethylene glycol-L-asparaginase. Drug Metab Dispos, 14:349-52, 1986.
-
MacEwen EG; Rosenthal R; Matus R; Viau AT; Abuchowski A. A preliminary study on the evaluation of asparaginase. Polyethylene glycol conjugate against canine malignant lymphoma. Cancer, 59:2011-5, 1987.
-
Ho DH; Wang CY; Lin JR; Brown N; Newman RA; Krakoff IH. Polyethylene glycol-L-asparaginase and L-asparaginase studies in rabbits. Drug Metab Dispos, 16:27-9, 1988.
-
Teske E; Rutteman GR; van Heerde P; Misdorp W. Polyethylene glycol-L-asparaginase versus native L-asparaginase in canine non-Hodgkin's lymphoma. Eur J Cancer, 26:891-5, 1990.
-
Aguayo A; Cortes J; Thomas D; Pierce S; Keating M; Kantarjian H. Combination therapy with methotrexate, vincristine, polyethylene-glycol conjugated-asparaginase, and prednisone in the treatment of patients with refractory or recurrent acute lymphoblastic leukemia. Cancer, 86:1203-9, 1999.
-
Tan Y; Sun X; Xu M; An Z; Tan X; Han Q; Miljkovic DA; Yang M; Hoffman RM. Polyethylene glycol conjugation of recombinant methioninase for cancer therapy. Protein Expr Purif, 12:45-52, 1998.
-
Hershfield MS; Buckley RH; Greenberg ML; Melton AL; Schiff R; Hatem C; Kurtzberg J; Markert ML; Kobayashi RH; Kobayashi AL; Abuchowski A. Treatment of adenosine deaminase deficiency with polyethylene glycol- modified adenosine deaminase. N Engl J Med, 316:589-96, 1987.
-
Levy Y; Hershfield MS; Fernandez-Mejia C; Polmar SH; Scudiery D; Berger M; Sorensen RU. Adenosine deaminase deficiency with late onset of recurrent infections: response to treatment with polyethylene glycol-modified adenosine deaminase. J Pediatr, 113:312-7, 1988.
-
Bory C; Boulieu R; Souillet G; Chantin C; Rolland MO; Mathieu M; Hershfield M. Comparison of red cell transfusion and polyethylene glycol-modified adenosine deaminase therapy in an adenosine deaminase-deficient child: measurement of erythrocyte deoxyadenosine triphosphate as a useful tool. Pediatr Res, 28:127-30, 1990.
-
Nishimura H; Matsushima A; Inada Y. Improved modification of yeast uricase with polyethylene glycol, accompanied with nonimmunoreactivity towards anti-uricase serum and high enzymic activity. Enzyme, 26:49-53, 1981.
-
Nishimura H; Ashihara Y; Matsushima A; Inada Y. Modification of yeast uricase with polyethylene glycol: disappearance of binding ability towards anti-uricase serum. Enzyme, 24:261-4, 1979.
-
Abuchowski A; Karp D; Davis FF. Reduction of plasma urate levels in the cockerel with polyethylene glycol-uricase. J Pharmacol Exp Ther, 219:352-4, 1981.
-
Fujita T; Yasuda Y; Takakura Y; Hashida M; Sezaki H. Tissue distribution of 111In-labeled uricase conjugated with charged dextrans and polyethylene glycol. J Pharmacobiodyn, 14:623-629, 1991.
-
Yasuda Y; Fujita T; Takakura Y; Hashida M; Sezaki H. Biochemical and biopharmaceutical properties of macromolecular conjugates of uricase with dextran and polyethylene glycol. Chem Pharm Bull, 38:2053-2056, 1990.
-
Nucci ML; Olejarczyk J; Abuchowski A. Immunogenicity of polyethylene glycol-modified superoxide dismutase and catalase. J Free Radic Biol Med, 2:321-5, 1986.
-
Olson NC; Grizzle MK; Anderson DL. Effect of polyethylene glycol-superoxide dismutase and catalase on endotoxemia in pigs. J Appl Physiol, 63:1526-32, 1987.
-
Liu TH; Beckman JS; Freeman BA; Hogan EL; Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol, 256:H589-93, 1989.
-
LeBlanc MH; Vig V; Randhawa T; Smith EE; Parker CC; Brown EG. Use of polyethylene glycol-bound superoxide dismutase, polyethylene glycol-bound catalase, and nimodipine to prevent hypoxic ischemic injury to the brain of newborn pigs. Crit Care Med, 21:252-9, 1993.
-
Abuchowski A; McCoy JR; Palczuk NC; van Es T; Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem, 252:3582-6, 1977.
-
Mossman BT; Marsh JP; Sesko A; Hill S; Shatos MA; Doherty J; Petruska J; Adler KB; Hemenway D; Mickey R; Vacek P; Kagan E. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis, 141:1266-71, 1990.
-
Pyatak PS; Abuchowski A; Davis FF. Preparation of a polyethylene glycol: superoxide dismutase adduct, and an examination of its blood circulation life and anti-inflammatory activity. Res Commun Chem Pathol Pharmacol, 29:113-27, 1980.
-
Tamura Y; Chi LG; Driscoll EM, Jr.; Hoff PT; Freeman BA; Gallagher KP; Lucchesi BR. Superoxide dismutase conjugated to polyethylene glycol provides sustained protection against myocardial ischemia/reperfusion injury in canine heart. Circ Res, 63:944-59, 1988.
-
Tanaka M; Stoler RC; FitzHarris GP; Jennings RB; Reimer KA. Evidence against the "early protection-delayed death" hypothesis of superoxide dismutase therapy in experimental myocardial infarction. Polyethylene glycol-superoxide dismutase plus catalase does not limit myocardial infarct size in dogs. Circ Res, 67:636-44, 1990.
-
Ooiwa H; Stanley A; Felaneous-Bylund AC; Wilborn W; Downey JM. Superoxide dismutase conjugated to polyethylene glycol fails to limit myocardial infarct size after 30 min ischemia followed by 72 h of reperfusion in the rabbit. J Mol Cell Cardiol, 23:119-25, 1991.
-
Haun SE; Kirsch JR; Helfaer MA; Kubos KL; Traystman RJ. Polyethylene glycol-conjugated superoxide dismutase fails to augment brain superoxide dismutase activity in piglets. Stroke, 22:655-9, 1991.
-
Rhee P; Waxman K; Clark L; Tominaga G; Soliman MH. Superoxide dismutase polyethylene glycol improves survival in hemorrhagic shock. Am Surg, 57:747-50, 1991.
-
Yoshida K; Burton GF; McKinney JS; Young H; Ellis EF. Brain and tissue distribution of polyethylene glycol-conjugated superoxide dismutase in rats. Stroke, 23:865-9, 1992.
-
Muizelaar JP; Marmarou A; Young HF; Choi SC; Wolf A; Schneider RL; Kontos HA. Improving the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: a phase II trial. J Neurosurg, 78:375-82, 1993.
-
Tang G; White JE; Gordon RJ; Lumb PD; Tsan MF. Polyethylene glycol-conjugated superoxide dismutase protects rats against oxygen toxicity. J Appl Physiol, 74:1425-31, 1993.
-
Muizelaar JP. Clinical trials with Dismutec (pegorgotein; polyethylene glycol- conjugated superoxide dismutase; PEG-SOD) in the treatment of severe closed head injury, in Armstrong D (ed), Free Radicals in Diagnostic Medicine. A System Approach to Laboratory Technology, Clinical Correlations, and Antioxidant Therapy. Plenum Press, New York, pp 389-400, 1994.
-
Mihara K; Oka Y; Sawai K; Takakura Y; Hashida M. Improvement of therapeutic effect of human recombinant superoxide dismutase on ischemic acute renal failure in the rat via cationization and conjugation with polyethylene glycol. J Drug Targeting, 2:317-321, 1994.
-
Morpurgo E; Cadrobbi R; Morpurgo M; Rigotti P; Schiavon F; Schiavon O; Caliceti P; Ancona E; Veronese FM. Protective effect of superoxide dismutase and polyethylene glycol- linked superoxide dismutase against renal warm ischemia/ reperfusion injury. Transplantation, 62: 1221-3, 1996.
-
Rocca M; Giavaresi G; Caliceti P; Veronese FM; Giardino R. Pathophysiological and histomorphological evaluation of polyacryloylmorpholine vs polyethylene glycol modified superoxide dismutase in a rat model of ischaemia/reperfusion injury. Int J Artif Organs, 19:730-4, 1996.
-
Rajagopalan S; Gonias SL; Pizzo SV. A nonantigenic covalent streptokinase-polyethylene glycol complex with plasminogen activator function. J Clin Invest, 75:413-9, 1985.
-
Brucato FH; Pizzo SV. Catabolism of streptokinase and polyethylene glycol-streptokinase: evidence for transport of intact forms through the biliary system in the mouse. Blood, 76:73-9, 1990.
-
Sakuragawa N; Shimizu K; Kondo K; Kondo S; Niwa M. Studies on the effect of PEG-modified urokinase on coagulation- fibrinolysis using beagles. Thromb Res, 41:627-35, 1986.
-
Kajihara J; Shibata K; Nakano Y; Nishimuro S; Kato K. Physicochemical characterization of PEG-PPG conjugated human urokinase. Biochim Biophys Acta, 1199:202-8, 1994.
-
Kajihara J; Shibata K; Kato K. Increased stability of PEG-PPG conjugated human urokinase against autolysis. Biosci Biotechnol Biochem, 61:197-8, 1997.
-
Iwasaki K; Iwashita Y; Ikeda K; Uematsu T. Efficacy and safety of hemoglobin-polyethylene glycol conjugate (pyridoxalated polyethylene glycol hemoglobin) as an oxygen-carrying resuscitation fluid. Artif Organs, 10:470-4, 1986.
-
Iwasaki K; Iwashita Y. Preparation and evaluation of hemoglobin-polyethylene glycol conjugate (pyridoxalated polyethylene glycol hemoglobin) as an oxygen-carrying resuscitation fluid. Artif Organs, 10:411-6, 1986.
-
Song D; Olano M; Wilson DF; Pastuszko A; Tammela O; Nho K; Shorr RG. Comparison of the efficacy of blood and polyethylene glycol-hemoglobin in recovery of newborn piglets from hemorrhagic hypotension: effect on blood pressure, cortical oxygen, and extracellular dopamine in the brain. Transfusion, 35:552-8, 1995.
-
Conover CD; Linberg R; Shum KL; Shorr RG. The ability of polyethylene glycol conjugated bovine hemoglobin (PEG- Hb) to adequately deliver oxygen in both exchange transfusion and top- loaded rat models. Artif Cells Blood Substit Immobil Biotechnol, 27:93-107, 1999.
-
Linberg R; Conover CD; Shum KL; Shorr RG. Increased tissue oxygenation and enhanced radiation sensitivity of solid tumors in rodents following polyethylene glycol conjugated bovine hemoglobin administration. In Vivo, 12:167-73, 1998.
-
Katre NV; Knauf MJ; Laird WJ. Chemical modification of recombinant interleukin 2 by polyethylene glycol increases its potency in the murine Meth A sarcoma model. Proc Natl Acad Sci U S A, 84:1487-91, 1987.
-
Yang JC; Schwarz SL; Perry-Lalley DM; Rosenberg SA. Murine studies using polyethylene glycol-modified recombinant human interleukin 2 (PEG-IL-2): antitumor effects of PEG-IL2 alone and in combination with adoptive cellular transfer. Lymphokine Cytokine Res, 10:475-80, 1991.
-
Meyers FJ; Paradise C; Scudder SA; Goodman G; Konrad M. A phase I study including pharmacokinetics of polyethylene glycol conjugated interleukin-2. Clin Pharmacol Ther, 49:307-13, 1991.
-
Mattijssen V; Balemans LT; Steerenberg PA; De Mulder PH. Polyethylene-glycol-modified interleukin-2 is superior to interleukin-2 in locoregional immunotherapy of established guinea-pig tumors. Int J Cancer, 51:812-7, 1992.
-
Bukowski RM; Young J; Goodman G; Meyers F; Issell BF; Sergi JS; McLain D; Fyfe G; Finke J. Polyethylene glycol conjugated interleukin-2: clinical and immunologic effects in patients with advanced renal cell carcinoma. Invest New Drugs, 11:211-7, 1993.
-
Bernsen MR; Dullens HF; Den Otter W; Heintz PM. Reevaluation of the superiority of polyethylene glycol-modified interleukin-2 over regular recombinant interleukin-2. J Interferon Cytokine Res, 15:641-5, 1995.
-
Yang JC; Topalian SL; Schwartzentruber DJ; Parkinson DR; Marincola FM; Weber JS; Seipp CA; White DE; Rosenberg SA. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer, 76:687-94, 1995.
-
Teppler H; Kaplan G; Smith KA; Montana AL; Meyn P; Cohn ZA. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin 2 in patients with human immunodeficiency virus type 1 infection. J Exp Med, 177:483-92, 1993.
-
Carr A; Emery S; Lloyd A; Hoy J; Garsia R; French M; Stewart G; Fyfe G; Cooper DA. Outpatient continuous intravenous interleukin-2 or subcutaneous, polyethylene glycol-modified interleukin-2 in human immunodeficiency virus-infected patients: a randomized, controlled, multicenter study. Australian IL-2 Study Group. J Infect Dis, 178:992-9, 1998.
-
Ramachandran R; Katzenstein DA; Winters MA; Kundu SK; Merigan TC. Polyethylene glycol-modified interleukin-2 and thymosin alpha 1 in human immunodeficiency virus type 1 infection. J Infect Dis, 173:1005-8, 1996.
-
Wood R; Montoya JG; Kundu SK; Schwartz DH; Merigan TC. Safety and efficacy of polyethylene glycol-modified interleukin-2 and zidovudine in human immunodeficiency virus type 1 infection: a phase I/II study. J Infect Dis, 167:519-25, 1993.
-
Teppler H; Kaplan G; Smith K; Cameron P; Montana A; Meyn P; Cohn Z. Efficacy of low doses of the polyethylene glycol derivative of interleukin-2 in modulating the immune response of patients with human immunodeficiency virus type 1 infection. J Infect Dis, 167:291-8, 1993.
-
Hillman RS. Hematopoietic agents, in Hardman JG: Limbird LE: Molinoff PB: Rudden RW: Goodman Gilman A (eds.), Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill, New York, pp 1311-1340, 1996.
-
Tanaka H; Satake-Ishikawa R; Ishikawa M; Matsuki S; Asano K. Pharmacokinetics of recombinant human granulocyte colony-stimulating factor conjugated to polyethylene glycol in rats. Cancer Res, 51:3710-4, 1991.
-
Ishikawa M; Okada Y; Satake-Ishikawa R; Kakitani M; Kawagishi M; Matsuki S; Kusaka M; Asano K. Pharmacological effects of recombinant human granulocyte colony- stimulating factor modified by polyethylene glycol on anticancer drug- induced neutropenia in mice. Gen Pharmacol, 25:533-7, 1994.
-
Bowen S; Tare N; Inoue T; Yamasaki M; Okabe M; Horii I; Eliason JF. Relationship between molecular mass and duration of activity of polyethylene glycol conjugated granulocyte colony-stimulating factor mutein. Exp Hematol, 27:425-32, 1999.
-
Satake-Ishikawa R; Ishikawa M; Okada Y; Kakitani M; Kawagishi M; Matsuki S; Asano K. Chemical modification of recombinant human granulocyte colony- stimulating factor by polyethylene glycol increases its biological activity in vivo. Cell Struct Funct, 17:157-60, 1992.
-
Yamasaki M; Asano M; Yokoo Y; Okabe M. Effect of divalent polyethylene glycol units, conjugated on human granulocyte colony-stimulating factor, on biological activities in vitro and in vivo. Drugs Exp Clin Res, 24:191-6, 1998.
-
Malik F; Delgado C; Knusli C; Irvine AE; Fisher D; Francis GE. Polyethylene glycol (PEG)-modified granulocyte-macrophage colony- stimulating factor (GM-CSF) with conserved biological activity. Exp Hematol, 20:1028-35, 1992.
-
Knusli C; Delgado C; Malik F; Domine M; Tejedor MC; Irvine AE; Fisher D; Francis GE. Polyethylene glycol (PEG) modification of granulocyte-macrophage colony stimulating factor (GM-CSF) enhances neutrophil priming activity but not colony stimulating activity. Br J Haematol, 82:654-63, 1992.
-
Norman PS; King TP; Alexander JF, Jr.; Kagey-Sobotka A; Lichtenstein LM. Immunologic responses to conjugates of antigen E in patients with ragweed hay fever. J Allergy Clin Immunol, 73:782-9, 1984.
-
Nishimura H; Takahashi K; Sakurai K; Fujinuma K; Imamura Y; Ooba M; Inada Y. Modification of batroxobin with activated polyethylene glycol: reduction of binding ability towards anti-batroxobin antibody and retention of defibrinogenation activity in circulation of preimmunized dogs. Life Sci, 33:1467-73, 1983.
-
Kamisako T; Miyawaki S; Gabazza EC; Ishihara T; Kamei A; Kawamura N; Adachi Y. Polyethylene glycol-modified bilirubin oxidase improves hepatic energy charge and urinary prostaglandin levels in rats with obstructive jaundice. J Hepatol, 29:424-9, 1998.
-
Muller U; Rabson AR; Bischof M; Lomnitzer R; Dreborg S; Lanner A. A double-blind study comparing monomethoxy polyethylene glycol-modified honeybee venom and unmodified honeybee venom for immunotherapy. I. Clinical results. J Allergy Clin Immunol, 80:252-61, 1987.
-
Nieforth KA; Nadeau R; Patel IH; Mould D. Use of an indirect pharmacodynamic stimulation model of MX protein induction to compare in vivo activity of interferon alfa-2a and a polyethylene glycol-modified derivative in healthy subjects. Clin Pharmacol Ther, 59:636-46, 1996.
-
Kita Y; Rohde MF; Arakawa T; Fagin KD; Fish EN; Banerjee K. Characterization of a polyethylene glycol conjugate of recombinant human interferon-gamma. Drug Des Deliv, 6:157-67, 1990.
-
Inoue H; Kadoya T; Kabaya K; Tachibana K; Nishi N; Sato M; Ohsawa M; Mikayama T; Mori KJ. A highly enhanced thrombopoietic activity by monomethoxy polyethylene glycol-modified recombinant human interleukin-6. J Lab Clin Med, 124:529-36, 1994.
-
Berger H, Jr.; Pizzo SV. Preparation of polyethylene glycol-tissue plasminogen activator adducts that retain functional activity: characteristics and behavior in three animal species. Blood, 71:1641-7, 1988.
- Abuchowski A; Davis FF. Preparation and properties of polyethylene glycol-trypsin adducts. Biochim Biophys Acta, 578:41-6, 1979.
Corresponding author: Reza Mehvar, Ph.D., School of Pharmacy, Texas Tech University Health Sciences Center, 1300 Coulter, Amarillo, TX 79106
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps