J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(1):114-124, 2000
A new method for one-step, no-carrier-added synthesis of cholesteryl 4-[18F]-fluorobenzoate ([18F]-CFB), a radiotracer used in detection of adrenal malignancies
Manuscript received November 30th, 1999, Revised February 14th, 2000; Accepted March 16th, 2000.
Amir
Reza Jalilian1, Hossein Afarideh,
Reza Najafi
Cyclotron
Department, Nuclear Research Center for Agriculture & Medicine
(NRCAM), Atomic Energy Organization of Iran (AEOI),
Karaj, Iran
Abbas
Shafiee, Maria Bineshmarvasti
Department
of Chemistry, Faculty of Pharmacy, Tehran University of Medical Sciences,
Tehran, Iran
PDF version for printing
Abstract
Purpose: Cholesteryl 4-[18F]-fluorobenzoate, a potential radiotracer used for adrenal and ovarian imaging, was prepared in no-carrier-added form from cholesteryl 4-N,N,N-trimethylanilinium trifluoromethanesulfonate.Method: The reaction was performed in one step using Kryptofix2.2.2/[18F], carbonate as the counter ion and dimethyl sulfoxide as the solvent at 110°C. Purification was performed using commercially available C18 and Si Sep-Paks.
Results: Column purification afforded the desired compound in 75-85 % radiochemical yield (EOS) with a specific activity about 74 KBq/mmole in about 20 minutes, with greater than 95% radiochemical and chemical purity (HPLC and TLC analysis).
Conclusion: This compound was prepared through a novel method which can be easily performed at distant locations from the main radionuclide production centers using Sep-Paks. The biodistribution of this compound in mice was confirmed to be similar to that reported in the literature.
Introduction
Cholesterol derivatives accumulate in steroid secreting tissues, to be used as the precusors in steroid hormones. Gamma emitting isotopes of iodine (131I t1/2=8 d or 123I t1/2=13 hrs) have been used to label cholesterol for administration to patients. These labeled compounds localize selectively in adrenal and ovarian tissue. Thus, cholesteryl esters are used as adrenal imaging agents to diagnose various adrenal diseases (1, 2).
Positron emitters were shown to have advantages over single photon emitters for several reasons. For example, Positron Emission Tomography (PET) isotopes are available for the basic elements of organic chemistry such as nitrogen, carbon, or oxygen, so that physiologic tracer substances can be synthesized to study perfusion and metabolism. The use of coincidence electronics for the detection of radiation aids in electronic collimation and contributes towards high image resolution. Photon attenuation is corrected individually for each patient using transmission images, so that quantitative measurements can be obtained (3).
Fluorine-18 is a suitable positron emitting isotope due to its low positron energy (0.64 MeV), low tissue range (2.4 mm), suitable half life (110 min) and steric similarity to the hydrogen atom (van der Waals radius: 1.2 Ĺ for H and 1.35 Ĺ for F) in comparison with iodine isotopes. Research in PET radionuclide production and subsequent labelling of new compounds has led to many clinical applications (4). Recently, a fluorine-18 labeled cholesteryl ester, [18F]-cholesteryl 4-fluorobezoate ([18F]-CFB), has been prepared and evaluated as a PET radioligand by a fluoro- for nitro- exchange reaction with 70-83% of incorporation of fluorine-18 by microwave heating (5). The purification of their product was accomplished by HPLC.
We now report a new and more facile method for synthesis of the [18F]-CFB by a fluoro exchange of the quaternary amine. This method consists of a purification procedure using two Sep-Pak columns instead of less reproducible microwave oven synthesis and HPLC purification (6). Automated synthesis units can be designed using our method at distant locations from the main PET production center where HPLC units may not exist.
Materials and Methods
All chemicals were purchased from the Aldrich Chemical Co., UK. 1H NMR spectra were obtained on a Bruker FT-80 (80 MHz) or a Varian (400 MHz) instrument with tetramethylsilane as the internal standard. Infrared spectra were taken on a Perkin-Elmer 781 spectrometer (KBr disks). Mass spectra were recorded using a Finnigan Mat TSQ-70 spectrometer. Thin-layer chromatography (TLC) of non-radioactive products was performed on silica gel polymer-backed (F 1500/LS 254, 20 ´ 20 cm, TLC Ready Foils Schleicher & Schuellâ) or glass plates (25 ´ 35 cm, E-Merck). Acetonitrile and dimethyl sulfoxide (DMSO) used for the labeling step were of ``Sure-Seal™`` grade supplied by Aldrich. Analytical HPLC to determine specific activity was performed with a Shimadzu™ LC-10AT. The HPLC system was equipped with two detector systems, that included a flow scintillation analyzer (with Packard™-150TR) and UV-visible (Shimadzu) detector. The analytical column was a Si Kromasil 100, 5 mm 250 ´ 4.6 mm (M & W), Inchrom™ column. A mixture of acetonitrile-chloroform (65:35) was used as mobile phase at a flow rate of 2 mL/min (Rt=3.4 min). The specific activity of cholesteryl 4-[18F]-fluorobenzoate (6b) was calculated using a standard curve prepared for 6a. Radiochromatography was performed using a rotary motor coupled to a Canberraä Germanium detector (model GC1020-7500SL) using polymer-backed silica gel papers. Purification of 6b was performed using a short C18 Sep-Pakä column purchased from Waters. Melting points were determined on a Reichert-Jungä apparatus and are uncorrected. Elemental microanalyses were within ±0.4% of theoretical values for C, H, F and N.
Preparation
of 18F-potassium fluoride from 18O-water. Fluorine-18
anion was prepared by 18 MeV proton bombardment of an enriched 18O-water
sample (purity more than 95%, Cortec, France). The sample was held in an
all-silver target using a 30 MeV cyclotron at the Nuclear Research Center
for Agriculture and Medicine, Karaj, Iran (NRCAM). After recovery of 18O-water
using an anion exchange resin (Dowex), fluoride-18 anion was eluted with a
1% potassium carbonate solution. The eluted solution was directly used for
the 18F-labeling reaction.
Cholesteryl 4-dimethylamino benzoate (3). A mixture of 4-dimethylaminobenzoic acid 2 (165 mg, 1 mmole), cholesterol 1 (386 mg, 1 mmole) and dicyclohexyl carbodiimide (DCC) (206 mg, 1 mmole) in anhydrous dichloromethane (10 mL) was stirred vigorously for 24 hrs at room temperature. The mixture was filtered and the filtrate was evaporated. The residue obtained was crystallized from ethyl acetate-hexane to give 375 mg (70%) of 3 as a light colorless powder; m.p. 89-92 °C; IR (KBr) 1736 cm-1 (s, C=O). 1H NMR (CDCl3) d (ppm) 6.81-8.07 (m, 4H, aromatic), 5.29 (m, 1H, C6-H), 3.41-3.48 (m, 1H, C3-H), 3.08 (s, 6H, CH3), 0.67-2.56 (m, 43H, aliphatic). MS: m/z (%) 533 (M+, 76), 386 (51), 371 (100), 368 (69), 353 (66), 164 (46).
Cholesteryl 4-fluorobenzoate (6a). This product was prepared according to the procedure described above for the preparation of 3, using equimolar amounts of acid 7, cholesterol 1 and DCC in anhydrous dichloromethane and subsequent recrystallization from ethyl acetate-hexane. (65%) m.p. 179-182 °C; IR (KBr) 1652 cm-1 (s, C=O), 1241 (s, C-O). 1H NMR (CDCl3) d (ppm) 7.45-7.66 (m, 2H, aromatic), 6.95-7.21 (m, 2H, aromatic), 5.32-5.37 (m, 1H, C6-H), 3.41-3.48 (m, 1H, C3-H), 0.68-2.45 (m, 43H, aliphatic). MS: m/z (%) 508 (M+, 68), 386 (48), 371 (100), 368 (55), 353 (69), 140 (43).
Cholesteryl 4-N,N,N-trimethylanilinium trifluoromethane sulfonate (5). Compound 5 was synthesized by reaction of methyl trifluoromethanesulfonate 4 with 3 in anhydrous dichloromethane at room temperature according to the literature (7). Compound 4 (25 mL, 0.1 mmole) was added to a solution of 3 (53 mg, 0.1 mmole) in anhydrous dichloromethane (10 mL) in one portion. After stirring for 2 hrs, the mixture was filtered and the solid was crystallized from dichloromethane-ether mixture to give 60 mg (86%) of 5 as fine colorless crystals; m.p. 192-195 °C; IR (KBr) 1690 cm-1(s, C=O), 1269 (brs, CF3SO3), 1132 (C-O); 1H NMR (DMSO) d (ppm) 6.65-7.29 (m, 4H, aromatic), 5.35 (m, 1H, C5-H), 3.63 (s, 9H, N(CH3)3), 3.36 (m, 1H, C3-H), 0.55-2.43 (m, 43H, aliphatic). MS: m/z (%) 697 (M+, 67), 548 (54), 386 (55), 371 (100), 368 (74), 353 (67), 149 (81).
Fluorination of Cholesteryl 4-trimethylanilinium triflate (5). To a vial containing potassium fluoride (40 mg, 0.69 mmole) in water (0.5 mL) and Kryptofix2.2.2 (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8] hexacosane) (200 mg, 0.54 mmole) in acetonitrile (1 mL), 5 mL acetonitrile was added. The mixture was stirred and then dried under an argon stream. A second 5 mL aliquot of acetonitrile was added and dried again. The vial was cooled and a solution of 5 (153 mg, 0.22 mmole) in DMSO (5 mL) was added to the dried mixture. The vial was capped and heated at 110 °C for 0.5 hr. After cooling, the mixture was mixed with water (25 mL) passed through a C18 column and washed with diethyl ether (10 mL). The organic layer was dried over anhydrous sodium sulfate and purified by preparative silica gel TLC on glass using a mixture of CHCl3-EtOAc (85:15). The desired fluoride compound was separated (Rf = 0.28). The identity of the product was confirmed by comparison with an authentic sample of 6a prepared before.
Radiolabeling.
Cholesteryl 4-[18F]-fluorobenzoate (6b). Product 6b was prepared from 5 using a procedure similar to that used for the preparation of 6a. A volume of target solution eluted with 100 mL of a 1% potassium carbonate (1 mg, 7 mmole) solution, containing 6 mCi of activity was transferred to a 2 mL vial containing Kryptofix222 (10 mg, 0.027 mmole) and anhydrous acetonitrile (0.5 mL). The mixture was evaporated by slight heating under a stream of argon. Drying was repeated after addition of two more 0.5 mL aliquots of anhydrous acetonitrile. A mixture of 5 (7 mg, 0.01 mmole) in anhydrous DMSO (0.25 mL) was added to the dried [18F]-isotope and heated at 110°C for 15 min. The mixture was cooled in an ice bath and rapidly drained into a syringe containing water (5 mL). The mixture was passed through a C18 Sep-Pak column. The column was washed with diethyl ether (1 mL) and the eluted solution was passed through a short silica column. The radioactive solution was checked for radiochemical purity by developing one drop of the latter solution using a polymer-backed silica gel layer with chloroform as the mobile phase.
Administration of [18F]-cholesteryl 4-fluorobenzoate (6b) to mice
Mature female NMRI mice (Pasteur Institute, Iran) weighing 20-25g (n =10) were used in experiments. The animals were kept in groups of ten, in cages under constant temperature (24°C) and 12 hr light/dark schedule. They had free access to standard mouse diet and tapwater except during the experiment. On the day of the experiment, animals were transferred to individual cages randomly and allowed to acclimatize for 30 min before injection of radioligand. Ethinyl estradiol was suspended in a mixture of Tween 80 solution (0.5%) and carboxymethylcellulose solution (1%) and the mixture was well mixed. The animals were divided into 2 groups. In the first group of ten mice, ethinyl estradiol was injected subcutaneously in the thigh 2 hrs before the injection of 18F-CFB in order to increase cholesterol derivative uptake into adrenal and ovarians (8). The second group of ten (control animals) was given only 18F-CFB injected in the same manner. The dried active residue obtained according to the above procedure was dissolved in borate buffer (0.5 mL) with shaking and warming for 30 sec. Enhanced radioligand uptake was observed 30 min after ethinyl esteradiol injection as previously mentioned (9). After 1 h various tissues were dissected and proportional uptake of 6b in different organs was measured by a gamma detector.
Results
Our group is interested in labeling different precursors that are localized in different organs. Since fluorine-18 has a half-life of only 110 min, it is important that labeling methods employing this nuclide be fast, give high yields and involve minimal chemical steps. In this report, we optimized the reaction conditions similar to aromatic fluorination that was reported previously (7). Highly sterically hindered cholesteryl esters have been shown to be good diagnostic molecules, based on their slow rate of ester hydrolysis and high accumulation rate in steroid secreting tissues (2,10). In this article we report the successful new facile synthesis of cholesteryl 4-[18F]-fluorobenzoate 6b, utilizing cholesteryl 4-N,N,N-trimethylanilinium trifluoromethanesulfonate 5. The labile 4-N,N,N-trimethylammonium triflate salt constitutes a very suitable leaving group, since the triflate anion is not nucleophilic in comparison to K222/18F. There are advantages of the present method over that reported by Jonson et al. For example, most radiosynthesis methods used in radiopharmaceutical production utilize the purification of the desired compound by HPLC, but separation of 6b from the starting triflate salt is performed easily using short Sep-Pak columns due to the great difference in their polarities. Sep-Pak purification provides practicality in designing automated synthesis units located remotely from main PET production centers. In most nuclear medicine centers Sep-Pak purification is favored over HPLC.
Using a microwave oven can be a problem because reaction yield is basically varied in response to different factors. For instance, geometry of the sample, the medium of the reaction, the microwave intensity and the ionic strength of the mixture are very influential parameters (6). In our work, using a thermal reaction with our precursor was highly reproducible and free from variations, with a yield of 75-85 %.
A high specific activity was produced via displacement of -NMe3+ by the [18F]-fluoride ion (11). The radiolabeled target molecule 6b, was prepared as illustrated in the Scheme. In the first step, cholesteryl 4-dimethylamino benzoate ester 3 was prepared with a high purity by condensation of the related acid 2 with cholesterol 1 using DCC in anhydrous dichloromethane following by recrystallization from ethyl acetate-hexane.
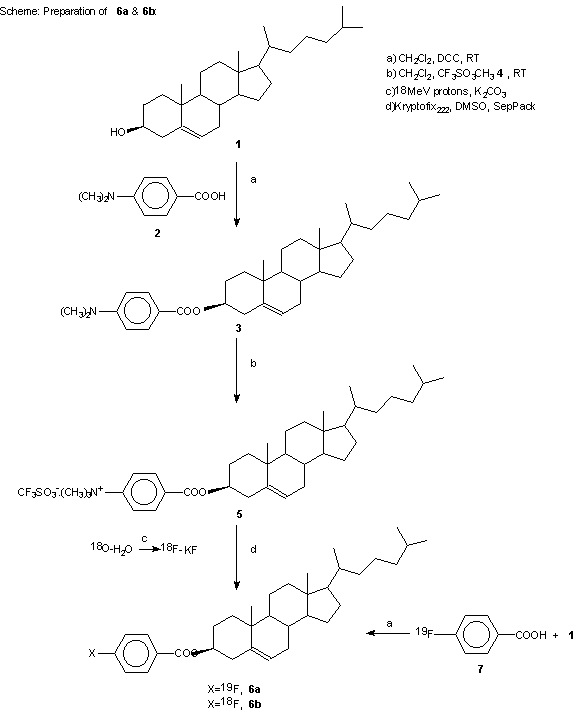
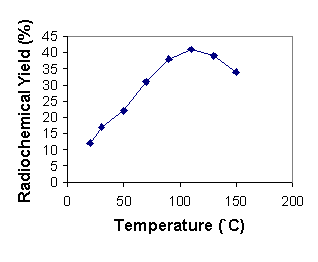
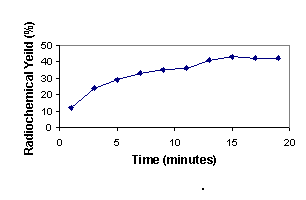
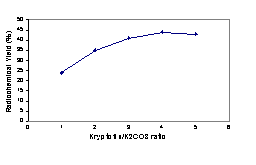
In order to produce a good leaving group on the benzene ring, methyl trifluoromethanesulfonate was treated with 3 in dichloromethane at room temperature according to the previous methods. It has been shown that the best counter ions for aromatic quaternary amine substrates are triflates (12). Activated aryl trimethylammonium triflate or perchlorate salts have been shown to be the best substrates for nucleophilic aromatic substitution by fluoride anion in the presence of the reactive aminopoly ether, Kryptofix 222, in an aprotic solvent (13). The methylation reaction with methyl trifluoromethanesulfonate is tolerant of a wide range of substituents on the aromatic ring. Total synthesis and purification of [18F]-CFB took about 20 minutes with a radiochemical yield of about 75% to 85% (decay corrected). Compound 5 has two active sites which are susceptible to nucleophilic attack. Higher temperature and an excess amount of base lead to hydrolysis of the ester group. Thus, in order to obtain optimal labeling reaction conditions, we performed many experiments to optimize temperature, time, solvent and the Kryptofix/base ratio. Heating the reaction mixture to 110°C increased the yield which then declined at 150°C. Further heating of the reaction mixture reduced the radiochemical yield due to decomposition or ester cleavage of precursor and/or product (Figure 1). At the optimum temperature, the reaction yield increased to a 15 min reaction time and then remained constant (Figure 2). DMSO was found to be the best aprotic solvent. On the other hand, tetrahydrofuran (THF) and dimethylacetamide (DMA) did not afford good yields because of the low solubility of the starting triflate salt 5 and Kryptate complex. Dimethylformamide (DMF) provided an intermediate result (Figure 3). Enhancing the Kryptofix to potassium carbonate ratio increased the radiochemical yield due to more available fluoride activity in solution (Figure 4). The carrier fluorine compound was prepared by two different pathways: a) the coupling reaction of 4-fluorobenzoic acid with cholesterol (in order to obtain an HPLC and radio TLC standard) and b) direct 19F-fluorination of 5 in order to ensure the radiosynthesis would be successful.
Figure 1: Yield of 6b as a function of temperature in DMSO (n=5) in 10 min
Direct [19F]-fluorination was performed by addition of 5 (dissolved in dimethylsulfoxide) to an azeotropic dried mixture of Kryptofix222 and potassium fluoride. The mixture was refluxed at 110 °C for 0.5 hr. Separation of the fluorine-19 compound was performed by silica gel chromatography on a glass plate, resulting in a colorless crystalline compound.
Figure 2: Yeild of 6b as a function of time at 110`C in DMSO (n=5)
Figure 3: Yield of 6b in 0.25 ml of different solvents at 110`C in 15 min (n=5).
Figure 4: Yield of 6b as a function of Kryptofix/K2CO3 ratio at 110`C in DMSO.
Characterization of the product was achieved by IR and 1H NMR spectroscopy and elemental analysis. We did not observe unlabeled products ([19F]-aryl fluorides or trimethylammonium salts), upon TLC or HPLC analysis of the final product. [18F]-CFB prepared from was examined by different chromatography methods repeatedly and carefully and had shown a consistent final specific activity in excess of 74 kBq/mmole (limit of detection) (Table 1). This value is consistent with the use of high specific activity, n.c.a [18F]-fluoride 6b. Furthermore, this indicated there was little (if any) dilution of the specific activity by [19F]-fluoride ion in the reagents or precursors, nor an exchange of the fluorines between the [18F]-fluoride ion and the trifluoromethyl group of the triflate counter ion.
Table 1: Effect of purification methods on yield of 6b:
|
Purification Method |
Radio-chemical Yield (%±SD) |
Radio-chemical Purity (%) |
Other
Detected Species
(%) |
None
|
74
±
6 (n=5) |
79 |
10-15 |
|
C18
Sep-Pak |
69
±5
(n=5) |
>96 |
None |
HPLC
|
71
±5
(n=5) |
97 |
None |
In conclusion, [18F]-CFB was prepared by a one-step procedure using no-carrier-added [18F]-fluoride and cholesteryl 4-N,N,N-trimethylanilinium trifluoromethane sulfonate salt 5 in high yield and high specific activity. The product was simply separated from unreacted salts by solid-liquid extraction using short columns with no apparent dilution of specific activity by fluoride ion. This is an efficient and rapid synthetic route to prepare fluorine-18 labeled substrates and can be easily automated to facilitate large scale syntheses of cholesteryl 4-[18F]-fluorobenzoate. The product can be obtained in greater than 95% radiochemical and chemical purity without application of HPLC.
Administration
of [18F]-cholesteryl 4-fluorobenzoate (6b) to mice
To confirm its biodistribution with current studies (14), [18F]-CFB was administered to mice that were pretreated with ethinyl estradiol. Pretreated mice were used to increase the adrenal uptake. Our results confirmed earlier reports like the observation of increased adrenal and ovarian uptake after administration of ethinyl estradiol (8). Spleen uptake increased by greater than 16 fold. Bone uptake was low in the control and pretreated groups implying the compound stability toward defluorination. Our results were in agreement with similar in vivo biodistribution experiments (14).
Acknowledgements
We gratefully acknowledge the financial support of the Iranian Atomic Energy Organization (IAEO), the technical assistance of the cyclotron team, and the editorial advice of Dr. G.G. Miller and Dr. S.A. McQuarrie.
References
-
van Dort, M., Santay, L. Schwendner, S.W., Potential tumor imaging agent 131I-radioiodinated sterol benzoates and carbamates, Int. J. Radiat. Appl. Inst. B, Nucl. Med. Biol. 16(6):603-608, 1989.
-
Seevers, R.H., Groziak, M.P., Wichert, J.P., Schwendner, S.W., Szabo S.M., Longino, M.A., Counsell, R.E., Tumor and organ imaging agents, 23; Sterol esters of iopanoic acid. J. Med. Chem. 25:1500-1507, 1982.
-
Porenta, G., Czernin, J., Schelbert, H., Positron emission tomography: principles and uses in cardiology. Wiener Medizinische Wochenschrift. 142(15-16):358-66, 1992.
-
Langstrom, B., Kihlberg, T., Bergstrom, M., Antoni, G., Bjorkman, M., Forngren, B.H., Forngren, T., Hartvig, P., Markides, K., Yngve, U., Ogren, M., Compounds labelled with short-lived beta(+)-emitting radionuclides and some applications in life sciences. The importance of time as a parameter. Acta Chemica Scandinavica. 53(9):651-69, 1999.
-
Jonson, S.D., Welch, M.J., Synthesis, biological evaluation, and baboon PET imaging of the potential adrenal imaging agent cholesteryl-p-[18F]fluorobenzoate. Nucl. Med. Biol. 26(1): 131-138, 1999.
-
Stoneelander, S., Elander, N., Microwave cavities: some parameters affecting their use in radiolabelling reactions. Int. J. Radiat. Appl. Instrum. Part A, Appl. Radiat. Isot. 42(9):885-887, 1991.
-
Haka, M.S., Kilbourne, M.R., Leonard, W. and Toorongian, S.A., Aryltrimethylammonium triflates as precursors to aryl [18F]fluorides: improved synthesis of [18F]GBR-13119. J. Labelled Compd. Radiopharm. 27: 823-828, 1989.
-
Counsell, R.E., Schwendner, S.W., Gross, M.D., Longino, M.A., McConnell, D.S., Lipoprotein incorporation enhances radioiodinated cholesteryl ester uptake into steroid hormone-secreting tissues. J. Nucl. Med. 30(6): 1088-1094, 1989.
-
Rudling, M.J., Role of the liver for the receptor-mediated catabolism of low-density lipoprotein in the 17-alpha-ethinylestradiol-treated rat. Biochim. Biophys. Acta. 919(2):175-180, 1987.
-
van Dort, M., Schwendner, S.W., Skinner, R.W., Gross, M.D., Counsell R.E., Potential tumor or organ-imaging agents, 24. Radioiodinated pregnenolone esters. Steroids. 44(1):85-93, 1984.
-
Angelini, G., Speranza, M., Wolf, A.P., Shiue, C.-Y., Nucleophilic aromatic substitution of activated cationic groups by 18F-labeled fluoride. A useful route to no-carrier-added (nca) 18F-labeled aryl fluorides. J. Fluorine Chem. 27: 177-183, 1985.
-
Shiue, C.Y., Fowler, J.S., Wolf, A.P., McPherson, D.W., Arnett, C.D., Zecca, L., No-carrier-added fluorine-18-labeled N-methylspiroperidol: synthesis and biodistribution in mice. J. Nucl. Med. 27(2):226-234,1986.
-
Kevill, D.N. and Shen, B.W., Perchlorate esters. 4. Kinetics and mechanism of the reactions of alkyl perchlorates with N,N-dimethylanilines in benzene. J. Am. Chem. Soc. 103: 4515-4521, 1981.
-
Jonson, S.D. and Welch, M.J., Cholesteryl [18F]p-fluorobenzoate as a new tumor radiotracer. XIIth International Symposium on Radiopharmaceutical Chemistry, Uppsala, Sweden, June 15-19, pp 823, 1997.
Corresponding author: Amir Reza Jalilian, Cyclotron Department, Nuclear Research Center for Agriculture & Medicine (NRCAM), Atomic Energy Organization of Iran (AEOI), P.O. Box: 31585-4395, Karaj, Iran.
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps