J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 3(2):228-233, 2000
Pharmaceutical interference with the [14C]carbon urea breath test for the detection of Helicobacter pylori infection
Manuscript received March 21st, 2000, Revised May 3rd, 2000; Accepted June 26th, 2000.
Douglas
N. Abrams1
Radiopharmacy,
Department of Radiology, Health Sciences Centre, Winnipeg, Manitoba, Canada
Ingrid
Koslowsky
Nuclear
Medicine, Diagnostic Imaging, Foothills
Medical Centre, Calgary, Alberta, Canada
Nuclear Medicine, Dept. of Diagnostic Radiology, Queen Elizabeth II Health Sciences Centre, Victoria General Site, Halifax, Nova Scotia, Canada
PDF version for printing
SUMMARY
Helicobacter pylori bacteria reside in the mucosal lining of the stomach where, due to a variety of factors, the infection predisposes patients to peptic ulcer disease. Detection of H. pylori is important in the treatment and follow-up of patients with peptic ulcer disease and the urea breath test is the method of choice. This article will briefly review the methods for diagnosing H. pylori, emphasizing the [14C]urea breath test. The agents which can interfere with the results of the breath test will be reviewed and the role of the consulting pharmacist will be discussed.
Introduction
The diagnosis and treatment of ulcers has changed considerably during the last two decades since Warren and Marshall implicated Helicobacter pylori in the genesis of peptic ulcer disease (1). Subsequently, there has been general agreement in the medical community that bacterial infection with H. pylori is causally related to cases of duodenal and gastric ulceration and to chronic gastritis (2-4). While approximately 30 to 50 % of asymptomatic controls culture positive for H. pylori, more than 90 % of patients presenting with duodenal or gastric ulcer are positive (5). Infected adults are at a 3.2 to 5.5 fold increased risk of developing peptic ulcer disease compared to the uninfected population, but the development of clinically significant sequelae seems to depend on the pathogenicity of the H pylori strain (5, 6). Eradicating H. pylori heals ulcers independent of other therapy and the addition of antibiotics to the therapeutic regimen has been shown to dramatically reduce the rate of ulcer recurrence. The Consensus Development Panel of the National Institutes of Health now recommends that all patients diagnosed with peptic or duodenal ulcers that are infected with H. pylori receive antimicrobial therapy (7).
Although most ulcer therapy provides excellent initial healing, the relapse rate between various regimens can differ significantly. The most effective therapy of H. pylori requires multiple drug treatment involving various combinations of antibiotics, histamine blockers, proton pump inhibitors and bismuth salt preparations (8, 9). In addition to potential classical drug interactions, the diagnostic tests used to confirm eradication are also subject to interference from many drugs. Therefore, the pharmacist has an important role in both the treatment and diagnosis of peptic ulcer disease.
H. pylori colonize the gastric epithelium in the stomach, causing acute inflammation progressing to superficial gastritis then to chronic atrophic gastritis. Acute infection leads to parietal cell dysfunction and acute hypochlorhydria, which increases gastrin and acid secretion. However the gastric pH soon returns to normal due to the release of bacterial urease which neutralizes the increased acid. This urease, which shows little difference between bacterial strains, is important in establishing chronic infection. The bacteria also induce a local inflammatory response attracting neutrophils, T cells, plasma cells and macrophages, but the bacteria, in their niche in the gastric mucosa, are not readily accessible to the antibodies produced. The H. pylori strains most involved in peptic ulcer also produce cytotoxins which promote the inflammatory response and reduce duodenal bicarbonate secretion (5).
Diagnosis
The presence of H. pylori can be diagnosed by different modalities which exploit various properties of the bacteria. These include endoscopy followed by histologic analysis, bacterial culture or urease testing of the biopsied sample, serologic detection of the antibody response, or detection of urease production by the carbon urea breath test (10).
Endoscopy with biopsy is the gold standard for detection of H. pylori, however, it is a relatively expensive and invasive procedure that is dependent on an experienced observer (10). Detection of the bacteria in a culture of the biopsy material is comparable to the precision of histologic examination of the specimen (specificity is 100 % for both procedures), but is more difficult to perform. The sensitivity (80 to 95 %) of histology is somewhat higher than tissue culture (sensitivity 60 to 90 %) but the need for multiple specimens to be examined by an experienced pathologist adds to the cost of endoscopy. Testing the biopsy sample for the presence of urease is a much simpler, quicker and less expensive test than either culture or histology and has high sensitivity (90 to 95 %) and specificity (98 to 100%). However, a biopsy sample is still required.
Serology tests detect the antibodies (determined by using radioimmunoassay techniques) produced in response to the infection and are useful to determine whether the patient has been exposed to H. pylori. The test has good specificity (98 to 100 %) and is the only procedure which is not influenced by prior antibiotic, bismuth compound, or omeprazole therapy. Unfortunately, a positive serology test can only presume current infection since the serum antibody titres to the bacteria decline slowly after eradication therapy (11). Therefore, long term follow up is required to ascertain the magnitude of this reduction limiting the use of serology in patient follow up post antibiotic treatment.
The [14C]carbon urea breath test is a sensitive, non-invasive test which can be used to determine the presence of H. pylori prior to initial treatment and for follow up after antibiotic therapy (12-15). The test is based on the detection of the enzyme urease, which is secreted by the bacteria to convert urea to ammonia. This produces an alkaline environment which facilitates their survival in the mucosal layer of the stomach. In the presence of urease, the [14C]urea is converted into [14C]bicarbonate and ammonium ions. The [14C]bicarbonate anion is absorbed into the blood stream, transported to the lungs, and subsequently exhaled as [14C]CO2. Detection of radioactive [14C]CO2 in the breath samples indicates an H. pylori infection, as other urease producing bacteria do not colonize the stomach (16).
The urea breath test can also be performed using non-radioactive 13C enriched urea (17). The disadvantage of using the 13C isotope is that a mass spectrometer is required for its detection. As this equipment is expensive and not readily available in most centres, 13C breath samples must typically be transported to another facility for analysis. Recent advances in laser (18) and infrared (19) spectroscopy show promise as lower cost replacements for mass spectroscopy.
In contrast, 14C can be readily detected using a liquid scintillation counter which is relatively inexpensive and not uncommon in hospital centres. In addition, the results can be available on the same day that the test is performed.
The urea breath test is a sensitive, specific, noninvasive and more cost effective test than endoscopy, and is the preferred method to check for eradication after therapy (20).
Patient Preparation
Patients should not be evaluated too soon following anti-ulcer therapy to avoid false negative test results. The bacterial load can be greatly reduced immediately following treatment with antibiotics, cytoprotectives and proton pump inhibitors (PPI’s). If the test for eradication is performed too soon, surviving bacteria that could re-colonize the stomach may not be detected.
Patients must fast for 4 to 6 hours prior to ingestion of the [14C]urea capsule or liquid. Any food in the stomach will dilute the product and delay excretion of the [14C]carbon dioxide. If the [14C]urea is administered as a liquid, the patients must rinse their mouths prior to ingestion to remove any urease producing commensal flora that may be present. Dentures should also be removed to avoid trapping of the fluid between the gums and dentures, which could result in a false positive test.
Testing Procedure
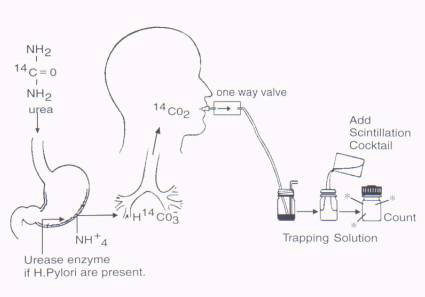
Prior to ingestion of [14C]urea, the patients provide a 'baseline' sample by blowing into a precisely titrated basic solution of benzethonium hydroxide (Hyamine hydroxide in methanol) containing an acid/base indicator such as phenolphthalein, bromothymol or thymolphthalein blue (Figure 1). This solution 'traps' the exhaled carbon dioxide by the formation of a carbamate and the liberation of hydrogen ions into the basic solution. When sufficient CO2 has been exhaled to convert all the benzethonium hydroxide to the carbamate, the solution becomes acidic and the indicator changes color. A capsule or liquid containing the [14C]urea is then swallowed and a series of breath samples are obtained at intervals up to 30 minutes after ingestion. The amount of radioactivity present in each sample represents the amount of [14C]CO2 exhaled which is proportional to the amount of [14C]urea metabolized by the bacteria. An alternative protocol can be performed by having the patient blow into a mylar balloon 10 minutes after swallowing a capsule containing the [14C]urea. The contents of the balloon are transferred via a pump into the trapping solution which is then processed as explained above (21).
Many protocols require multiple sampling to calculate the total amount of [14C]CO2 produced as described by Henze et al (13) who preferred the multiple sampling technique to generate a curve describing the fraction of the dose excreted as a function of time.(21).
Figure 1: Schematic representation of the detection of H. pylori infection using the [14C] Urea Breath Test.
The area under the curve was evaluated to calculate the total amount of [14C]CO2 excreted. The accuracy of a single sample taken at 10 or 20 minutes after ingestion of the radiolabelled urea (14,15) has recently been validated. Desroches et al (15) suggested using a 2 mmol solution of benzethonium hydroxide and a single breath sample obtained 20 minutes after ingestion of 5 mCi of [14C]urea. Excretion of more than 0.33 % of the ingested dose was considered positive for the presence of H. pylori. The sensitivity and specificity of the single breath protocol was 98 % and 100 % respectively.
Patient Radiation Burden
The ingested [14C]urea is rapidly excreted from the body as either [14C]CO2 in the breath or as intact [14C]urea in the urine (12). In uninfected individuals, it is estimated that up to 30 % of the radioactivity is excreted in the breath as [14C]CO2 with the remaining 70 % excreted by the kidneys unchanged. Respiratory excretion of [14C]CO2 is increased to 60 % in patients infected with H. pylori, with the remainder excreted in the urine as [14C]urea (12). The radiation exposure from a 1 mCi dose of 14C is estimated to be equivalent to the amount of radiation received by the patient from the natural environment over a period of 11 hours (22).
Pharmaceutical Interference with the Urea Breath Test
The [14C]urea breath test is dependent on the concentration of H. pylori present in the gut. Therefore any drug which suppresses the growth of bacteria can theoretically affect the results of the urea breath test. As well, other drugs commonly used in the treatment of ulcers can potentially interact with the test. Some of these drug-test interactions are well documented while others remain speculative. A summary of these interactions is provided in Table 1.
Table 1. Pharmaceuticals Interfering with the 14C Urea Breath Test
|
Class |
Pharmaceutical
(exceptions) |
Effect |
Recommendations |
References |
|
Antibiotics |
All
classes except: Vancomycin, Nalidixic
Acid, Trimethoprim, Amphotericin
B |
Inhibitory
or bactericidal activity |
Stop
medication 30 days prior to test |
24 |
|
Cytoprotectives |
Bismuth
Salts |
Bactericidal
effect. |
Stop
medication 30 days prior to test |
24-26 |
|
Proton
pump inhibitors |
Omeprazole,
Lansoprazole, Pantoprazole |
Bactericidal
or bacteriostatic activity. |
Stop
medication 7-14 days prior to test |
27 |
|
H2
Receptor Antagonists |
Cimetidine,
Famotidine, Nizatidine, Ranitidine |
May
promote urease producing oral flora. May promote H. pylori
proliferation. |
Stop
medication 12-24 hours prior to test |
2,3,12,24 |
|
Antacids |
All
products |
Decrease
in urease activity. May erroneously decrease result values. |
Not
contraindicated but should be reduced if possible |
29 |
In vitro studies have shown that a wide range of antibiotics possess varying inhibitory or bactericidal activity against H. pylori including beta-lactams, macrolides, nitrofurans, and quinolines, with the exception of vancomycin, nalidixic acid, trimethoprim, and amphotericin B (23, 24). In order to ensure that complete eradication of H. pylori has occurred, it is essential that antibiotic treatment for any disorder has been completed at least 30 days prior to testing for H. pylori.
Bismuth salts must also be discontinued for 30 days prior to the urea breath test. Bismuth has also been shown to inhibit H. pylori activity due to both its bactericidal activity and its cytoprotective effect (24-26). Electron microscopy of gastric biopsy specimens following treatment with bismuth demonstrated coating of the bacteria with the bismuth compound followed by swelling and lysis of the organism. It is also possible that bismuth inhibits bacterial enzymes which could disrupt cell metabolism, rendering the bacteria susceptible to the body's normal defenses (26).
Proton pump inhibitors such as omeprazole, lansoprazole, or pantoprazole have bactericidal activity against H. pylori in the early stages of infection, when the number of bacteria colonizing the gut is low. A bacteriostatic effect has been observed when bacterial densities are very large (27). Procedure guidelines for the urea breath test typically suggest discontinuing PPIs two weeks prior to testing (28). A one week hiatus from the drug may be appropriate considering that many patients rely on the use of PPIs to relieve the symptoms of gastritis or ulcer.
H2 receptor antagonists and sucralfate were found ineffective in the suppression of H. pylori (2,24). H2 receptor antagonists were found to heal the gastric wall, but left the bacteria adhering closely to the gastric mucosa. They are used sometimes to reduce the painful gastric symptoms experienced by patients who must stop PPIs prior to the urea breath test. However, H2 receptor antagonists have been implicated in the proliferation of H. pylori (23) and in the growth of urease producing commensal oral flora due to an increase in gastric pH (12). For these reasons, patients are often requested to stop taking H2 receptor antagonists for 12 to 24 hours prior to the urea breath test.
Antacids have also been shown to suppress bacterial growth for a short period of time after administration. Berstad et al reported a 30% decrease in urease activity immediately following treatment with antacids (29) but the urease activity rebounded to pretreatment levels within 2 weeks. Despite the reduction, the urease activity immediately post antacid treatment was still significantly higher than in those individuals with no evidence of H. pylori infection. Consequently, antacids are not typically contraindicated prior to performing the [14C]carbon urea breath test.
Conclusion
Treatment to eradicate H. pylori has dramatically changed the natural history of peptic ulcer disease from a chronic, relapsing disorder to a simple, one treatment cure. The [14C]carbon urea breath test is a easy, non-invasive test that accurately predicts the presence of H. pylori in affected patients. In addition to counselling for standard potential adverse medication effects and interactions, physicians, pharmacists, and patients should also be aware that any medication that temporarily suppresses these bacteria should be discontinued prior to performing the urea breath test in order to increase the reliability of the results.
Acknowledgement
The authors would like to thank Ms. Rosina Kanerva, Foothills Medical Centre for illustrating Figure 1.
References
-
Marshall BJ; Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet, 1:1311-1315, 1984.
-
Rauws EAJ; Langenberg W; Houthoff HJ; Zanen HC; Tytgat GNJ. Campylobacter pyloridis associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology, 94:33-40, 1988.
-
Peterson WL. Helicobacter pylori and peptic ulcer disease. N Engl J Med, 324:1043-1048, 1991.
-
Fennerty MB. Helicobacter pylori. Arch Intern Med, 154:721-727, 1994.
-
Peek RM.; Blaser MJ. Pathophysiology of Helicobacter pylori-induced gastritis and peptic ulcer disease. Am J Med, 102: 200-207, 1997.
-
Atherton JC; Peek RM; Tham KT; Cover TL; Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology, 112: 92-99, 1997.
-
NIH Consensus Development Panel. Helicobacter pylori in peptic ulcer disease. JAMA, 272:65-69, 1994.
-
Graham DY; Lew GM; Klein PD; Evans DG; Evans DJ Jr.; Saeed ZA; Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med, 116:705-708, 1992.
-
Sung JJ; Chung SC; Ling TK; Yung MY; Cheng AF; Hosking SW; Li AK. One-year follow-up of duodenal ulcers after 1-wk triple therapy for Helicobacter pylori. Am J Gastroenterol, 89(2):199-202, 1994.
-
Brown KE; Peura DA. Diagnosis of Helicobacter pylori infection. Gastroenterol Clin North Am, 22:105-115,1993.
-
Kosunen TU; Seppala K; Sarna S; Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet, 339:893-895, 1992.
-
Marshall BJ; Surveyor I. Carbon-14 urea breath test for the diagnosis of Campylobacter pylori associated gastritis. J Nucl Med, 29:11-16,1988.
-
Henze E; Malfertheiner P; Clausen M; Burkhardt H; Adam WE. Validation of a simplified carbon-14-urea breath test for routine use for detecting Heliobacter pylori noninvasively. J Nucl Med, 31:1940-1944, 1990.
-
Debongnie JC; Pauwels S; Raat A; de Meeus Y; Haot J; Mainguet P. Quantification of Helicobacter pylori infection in gastritis and ulcer disease using a simple and rapid carbon-14-urea breath test. J Nucl Med, 32:1192-1198, 1991.
-
Desroches JJ; Lahaie RG; Picard M; Morais J; Dumont A; Gaudreau C; Picard D; Chartrand R. Methodological validation and clinical usefulness of carbon-14-urea breath test for documentation of presence and eradication of Helicobacter pylori infection. J Nucl Med, 38:1141-1145, 1997.
-
Hazell SL; Borody TJ; Gal A; Lee A. Campylobacter pyloridis gastritis I: Detection of urease as a marker of bacterial colonization and gastritis. Am J Gastroenterol, 82:292-296, 1987.
-
Dill S, Payne-James JJ; Misiewicz JJ; Grimble GK; McSwiggan D; Pathak K; Wood AJ; Scrimgeour CM; Rennie MJ. Evaluation of 13C-urea breath test in the detection of Helicobacter pylori and in monitoring the effect of tripotassium dicitratobismuthate in non-ulcer dyspepsia. Gut, 31:1237-1241, 1990.
-
Tanahashi T; Kodama T; Yamaoka Y; Sawai N; Tatsumi Y; Kashima K; Higashi Y; Sasaki Y. Analysis of the 13C-urea breath test for the detection of Helicobacter pylori infection based on the kinetics of delta-13CO2 using laser spectroscopy. J Gastorenterol Hepatol, 13(7):732-737, 1998.
-
Savarino V; Mela GS; Zentilin P; Bisso G; Pivari M; Mansi C; Mele MR; Bilari C; Vigneri S; Celle G. Comparison of isotope ratio mass spectrometry and nondispersive isotope-selective infrared spectroscopy for 13C-urea breath test. Am J Gastroenterol, 94: 1203-1208, 1999.
-
Rollan A; Giancaspero R; Arrese M; Figueroa C; Vollrath V; Schultz M; Duarte I; Vial P. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection after antibiotic treatment. Am J Gastroenterol, 92: 1268-1274, 1997.
-
Peura DA; Pambianco DJ; Dye KR; Lind C; Frierson HF; Hoffman SR; Combs MJ; Guilfoyle E; Marshall BJ. Microdose 14C-urea breath test offers diagnosis of Helicobacter pylori in 10 minutes. Am J Gastroenterol, 91:233-238, 1996.
-
Stubbs JB; Marshall BJ. Radiation dose estimates for the carbon-14-labelled urea breath test. J Nucl Med, 34:821-825, 1993.
-
McNulty CAM. The treatment of campylobacter-associated gastritis. Am J Gastroenterol, 82:245-247, 1987.
-
Goodwin CS; Blake P; Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrobial Chemother, 17:309-314,1986.
-
Holroyde MJ; Yeakle C; Pepple S. Gastric cytoprotection by bismuth subsalicylate. Gastroenterology, 86:1116 (abst),1984.
-
Wagstaff AJ; Benfield P; Monk JP. Colloidal bismuth subcitrate: A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic use in peptic ulcer disease. Drugs, 36:132-157, 1988.
-
Mirshahi F; Fowler G; Patel A; Shaw G. Omeprazole may exert both a bacteriostatic and a bacteriocidal effect on the growth of Helicobacter pylori (NCTC 11637) in vitro by inhibiting bacterial urease activity. J Clin Pathol, 51:220-224, 1998.
-
Balon H; Gold CA; Dworkin HJ; McCormick VA; Freitas JE. Procedure guideline for carbon-14-urea breath test. J Nucl Med, 39:2012-2014, 1998.
-
Berstad K; Weberg R; Berstad A. Suppression of gastric urease activity by antacids. Scand J Gastroenterol, 25:496-500,1990.
Corresponding author: D. N. Abrams, 820 Sherbrook Street, Radiopharmacy, GC 219, Health Sciences Centre, Winnipeg, Manitoba, Canada, R3A 1R9. Dabrams@exchange.hsc.mb.ca
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
http://www.ualberta.ca/~csps