J Pharm Pharmaceut Sci (www.ualberta.ca/~csps) 2 (2):53-61, 1999
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Polymeric Films As Vehicle For Buccal Delivery: Swelling, Mechanical, and Bioadhesive Properties. Received manuscript August 9th, 1999; Revised August 24th, 1999; Accepted August 31st, 1999 Kok Khiang Peh, Choy
Fun Wong1, PDF Version for printing Abstract Purpose. To investigate the suitability of an SCMC (sodium carboxymethyl cellulose/polyethylene glycol 400/carbopol 934P) and an HPMC (hydroxypropylmethyl cellulose/polyethylene glycol 400/carbopol 934P) films as drug vehicle for buccal delivery. Methods. The mechanical and in vitro bioadhesive strength properties of the films were investigated using texture analyzer equipment, while swelling behavior was studied in different media, namely, distilled water and simulated saliva solution. In addition, the in vivo bioadhesion of the film was studied by estimating the film residence time on buccal mucosa of human volunteers. Results. Increase in carbopol 934P content was found to elevate the elasticity, softness and bioadhesive strength but decrease the strength and degree of swelling of both SCMC and HPMC films. SCMC films swelled more extensively in distilled water while HPMC films in simulated saliva solution. HPMC films exhibited greater in vivo bioadhesion although the in vitro bioadhesive strength was lower than SCMC films. Correlation existed between the in vivo and in vitro bioadhesion data within the polymer, but no rank correlation was observed between the two polymers. Conclusion. HPMC films may be preferred over SCMC films as drug vehicle for buccal delivery as the former was tougher, more elastic, more bioadhesive in vivo and swelled in a more tolerable manner in the oral cavity than the latter. Introduction Buccal drug delivery has lately become an important route of drug administration. This route of drug administration has recently been extensively reviewed by Shojaei (1). Various bioadhesive mucosal dosage forms have been developed, which included adhesive tablets (2,3), gels (4,5), ointments (6), patches (7-9), and more recently films (10). The use of polymeric films for buccal delivery has not yet been widely investigated, although they have been extensively employed in pharmaceutical tablet coating formulations to protect tablet cores from environmental extremes, improve appearance, mask undesirable taste, and control the drug release (11). Buccal film may be preferred over adhesive tablet in terms of flexibility and comfort. In addition, they can circumvent the relatively short residence time of oral gels on the mucosa, which is easily washed away and removed by saliva (7,12). Moreover, the buccal film is able to protect the wound surface, thus reduce pain and also could treat oral diseases more effectively. An ideal buccal film should be flexible, elastic, soft yet adequately strong to withstand breakage due to stress from mouth activities. Moreover, it must also possess good bioadhesive strength so that it can be retained in the mouth for a desired duration. Swelling of film, if exists should not be too extensive to prevent discomfort. As such, the mechanical, bioadhesive, and swelling properties of buccal film are critical and essential to be evaluated. The objective of the present study was to investigate the suitability of SCMC and HPMC films as drug vehicle for buccal delivery. The films were evaluated in terms of mechanical, bioadhesive, and swelling properties. In addition, in vivo bioadhesion of the films was evaluated by measuring the film residence time on human buccal mucosa of human volunteers. Materials and Methods Materials Hydroxypropylmethyl cellulose (Methocel K15M, HPMC) was a gift from Colorcon, UK. Sodium carboxymethyl cellulose (Cekol 700, SCMC) was purchased from Metsa-Serla, Sweden. Poly(acrylic acid), carbopol 934P (CP) was a gift from BF Goodrich, Cleveland, USA. Polyethylene glycol 400 (PEG 400) was purchased from BDH Laboratory Supplies, Poole, UK. All the materials were used as received. Preparation of Drug Free Film Drug-free films containing different ratios of HPMC or SCMC to CP but constant proportion of PEG 400 were prepared. The ratios of HPMC or SCMC, PEG 400 and CP studied were 0.7:1.0:0.3, 0.5:1.0:0.5, and 0.3:1.0:0.7. A total of 1% w/v polymeric solution were allowed to stir for 6 h and stand overnight to remove all the air bubbles entrapped. The solutions were then casted onto a petri dish and dried in the oven at 60°C until completely dry. The film was carefully removed from the petri dish, checked for any imperfections and cut according to the size required for testing. The samples were stored in a glass container maintained at temperature of 24±1°C and relative humidity of 60±5% until further analysis. The thickness of each sample was measured using a micrometer (Digimatic Micrometer, Mitutoyo, Tokyo, Japan) at five locations (center and four corners), and the mean thickness calculated. Samples with air bubble, nicks or tears and having mean thickness variations of greater than 5% were excluded from analysis. Measurement of Mechanical Properties Mechanical properties of the films were evaluated using a TA.XT2 texture analyzer equipment equipped with a 5 kg load cell (Stable Micro Systems, Haslemere, Surrey, UK). Film strip in dimension of 50x10 mm and free from air bubbles or physical imperfections, were held between two clamps positioned at a distance of 3 cm. A cardboard was attached on the surface of the clamp via a double-sided tape to prevent the film from being cut by the grooves of the clamp. During measurement, the strips were pulled by the top clamp at a rate of 2.0 mm/s to a distance of 5 cm for the SCMC, and 10 cm for the HPMC films before returning to the starting point. The force and elongation were measured when the films broke. Results from film samples, which broke at and not between the clamps were not included in calculations. Measurements were run in four replicates for each film. The following equations were used to calculate the mechanical properties of the films:
Measurement of Film Swelling The film swelling studies were conducted using two media, namely, distilled water and simulated saliva solution which consisted of phosphate buffer saline solution (2.38 g Na2HPO4, 0.19 g KH2PO4 and 8.00 g NaCl per liter of distilled water adjusted with phosphoric acid to pH 6.75). Each film sample (surface area; 2.84 cm2) was weighed and placed in a preweighed stainless steel wire mesh with sieve opening of approximately 800 mm. The mesh containing the film sample was then submerged into 15 ml medium contained in a plastic container (diameter 5.00 cm, height 1.54 cm). Increase in weight of the film was determined at preset time intervals until a constant weight was observed. Each measurement was repeated four times. The degree of swelling was calculated using parameters, (Wt - Wo )/Wo, where Wt is the weight of film at time t, and Wo is the weight of film at time zero. In vitro Bioadhesive Strength Evaluation The in vitro bioadhesive strength of films was determined employing method described by Wong et al. (9) with slight modification. Bioadhesive strength measurement was conducted using a TA.XT2 texture analyzer equipment with fresh chicken pouch as the model tissue. The chicken pouch was placed on a foam tape mounted onto the cylindrical perspex support (2 cm diameter and 4 cm length) and secured with a string. The foam tape at the cross-sectional end of the perspex support was to provide cushioning effect. The whole perspex support was then positioned at the bottom of the measuring system and held in place by a clamp. Adhesive film cut in the size of cross-sectional area of the perspex support (diameter, 2 cm; surface area, 3.14 cm2) was affixed to another perspex support of similar dimension using a double sided tape. The perspex support was then screwed onto the upper probe of the instrument. The two perspex supports were aligned to ensure that the film comes into direct contact with the surface of the chicken pouch when the upper support was lowered. During measurement, 100 ml of simulated saliva fluid (2.38 g Na2HPO4, 0.19 g KH2PO4 and 8.00 g NaCl per liter of distilled water adjusted with phosphoric acid to pH 6.75) was evenly spread on the surface of the tissue. The film was lowered at a speed of 0.5 mm/s to contact with the tissue at a force of 1 N for a contact time of 10 s. It was then withdrawn at a rate of 1.0 mm/s to a distance of 10 mm. An acquisition rate of 200 points/s was chosen for the analysis. Data collection and calculation were performed using the XTRA Dimension software package of the instrument. Work of adhesion and peak detachment force were used to evaluate the bioadhesive strength of the films. The work of adhesion was calculated from the area under the force-distance curve, and the peak detachment force was taken as the maximum force needed for detaching the film from the tissue. Measurements were performed in triplicate. Residence Time Measurement Using Human Volunteers Ten healthy adult volunteers (3 male, 7 female), aged between 20 and 27 years old, participated in the study after signing informed consents. The study was conducted in accordance to the Declaration of Helsinki guidelines. Prior to the test, the volunteers were provided with training. They were required to rinse their mouth with distilled water before a piece of the film (surface area; 1 cm2) was placed on their buccal mucosa. The volunteers were refrained from food, drinks and talking during the evaluation period. The volunteers were asked to record the residence time of the film on buccal mucosa in the oral cavity, which was taken as the time for the film to dislodge completely from the buccal mucosa. Statistical Analysis The results obtained from the mechanical properties evaluation, in vitro bioadhesive strength evaluation and residence time measurement of the HPMC and SCMC films were analyzed statistically using one-way analysis of variance (13). When a statistically significant difference (p < 0.05) was obtained, Tukey HSD test was then performed. Results and Discussion Mechanical Properties The tensile testing gives an indication of the strength and elasticity of the film, reflected by the parameters, tensile strength (TS), elastic modulus (EM) and elongation at break (E/B). A soft and weak polymer is characterized by a low TS, EM and E/B; a hard and brittle polymer is defined by a moderate TS, high EM and low E/B; a soft and tough polymer is characterized by a moderate TS, low EM and high E/B; whereas a hard and tough polymer is characterized by a high TS, EM and E/B (14). Another parameter, Strain has been used as an indicator of the overall mechanical quality of the film (15). A high strain value indicates that the film is strong and elastic. Hence, it is suggested that a suitable buccal film should have a relatively high TS, E/B and Strain but a low EM. Table 1 depicts the mechanical
properties of various SCMC and HPMC film preparations. A statistically
significant difference was observed in all the parameters evaluated
within respective polymer type film. For SCMC films, increase in CP
content was Table 1. Mechanical properties of different SCMC and HPMC films (Mean ± SD, n = 4).
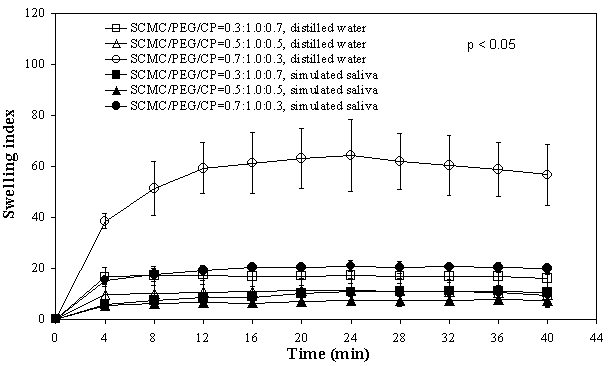
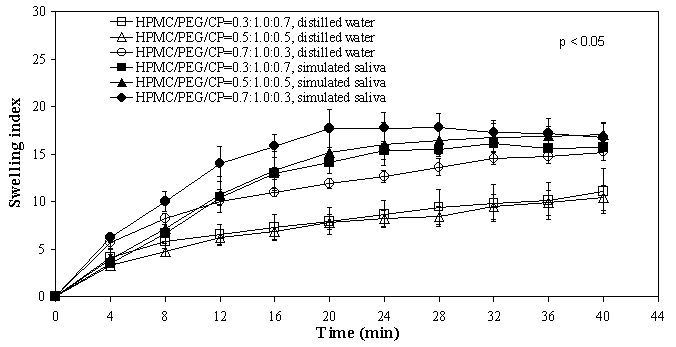
In comparison, the mean TS values of both HPMC and SCMC films were closely comparable for similar compositions, except between films containing 70% CP where the HPMC film demonstrated a significantly higher value. Increase in CP content rendered the HPMC films more elastic than SCMC films. Since HPMC (neutral polymer) and SCMC (anionic polymer) possessed different physical properties, incorporation of CP was expected to produce films with different mechanical properties. Swelling of films Figures 1 and 2 depict the degree of swelling of respective SCMC and HPMC films in both the distilled water and simulated saliva solution. Figure 1. Swelling versus time profiles of SCMC/PEG400/CP films indistilled water and simulated saliva solution Figure 2. Swelling versus time profiles of HPMC/PEG400/CP films in distilled water and simulated saliva solution Maximum swelling was seen with formulations containing high proportion of SCMC and HPMC (70%). Increasing CP content was found to reduce the extent of swelling of the films. It can be noted from the plots that the degree of swelling for SCMC films was higher in distilled water than in simulated saliva solution. Conversely, HPMC films swelled at a comparatively greater extent in the simulated saliva solution than in distilled water. These findings suggested that ionic strength and pH play an important role in affecting the swelling of HPMC and SCMC films. The effect of ionic strength and pH on the swelling of polymer has been described by Park and Robinson (16). The rate of swelling for SCMC films was comparatively faster than HPMC films. For instance, SCMC films achieved maximum/plateau in swelling at approximately 4-8 min, while HPMC films about 20-24 min in simulated saliva solution, indicating that SCMC films exhibited faster rate of water uptake and hydration than HPMC films. The swelling state of the polymer was reported to be crucial for its bioadhesive behaviour (17, 18). Adhesion occurs shortly after the beginning of swelling but the bond formed is not very strong (17). The adhesion will increase with the degree of hydration until a point where overhydration leads to an abrupt drop in adhesive strength due to disentanglement at the polymer/tissue interface. In vitro bioadhesion evaluations Table 2 depicts the mean values of in vitro bioadhesive strength of the films. A correspondent increase in the bioadhesion strength was observed with an increase in CP content, consistent with findings reported in the literature (19, 20). There was a statistically significant difference in both the work of adhesion (p = 0.016, SCMC; p = 0.020, HPMC) and the peak detachment force (p = 0.042, SCMC; p = 0.011, HPMC) between the three film preparations. Increasing CP content from 30% to 70% led to a significant increase in the work of adhesion and the peak detachment force of SCMC as well as HPMC films. However, no significant difference was observed in both the parameters between 30% and 50% CP. For SCMC films, the work of adhesion for films with 50% CP was significantly lower than films of 70% CP, although there was no significant difference in the peak detachment force. On the other hand, no significant difference was observed in both the work of adhesion and peak detachment force for HPMC films consisting of 50% and 70% CP. In general, SCMC films showed slightly higher bioadhesive strength values than HPMC films for similar compositions. Table 2. In vitro bioadhesive strength and buccal residence time of films (Mean ± SD).
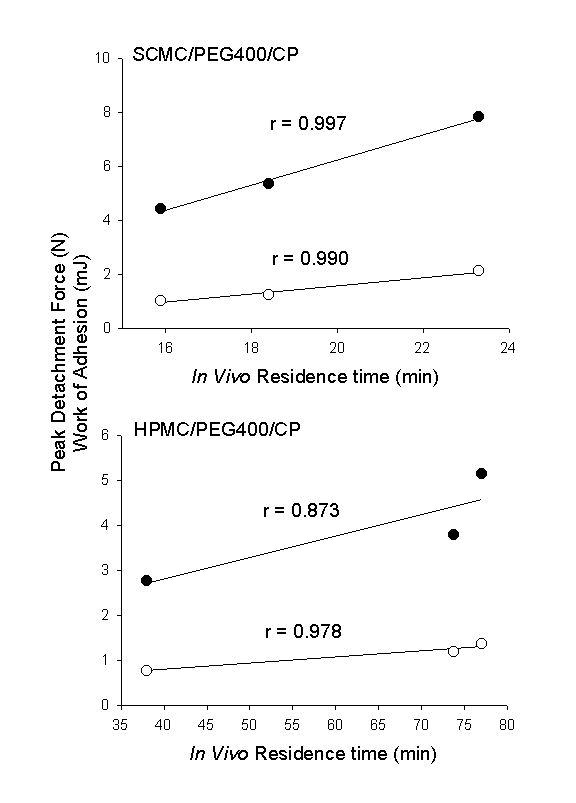
Various mechanisms have been proposed to explain the in vitro bioadhesion or mucoadhesion phenomena. These included electrical double layers, electrostatic attractions, hydrogen bonding, Van der Waals force, hydrophobic bonding, wetting, diffusion-interpenetration, physical entanglements, and surface free energy (16, 21, 22). SCMC is a polyanionic polymer bearing carboxylic groups. Its incorporation can increase the surface charge density of the films. Moreover, the carboxylic groups can form hydrogen bonds with the tissue. In addition, the SCMC films had a faster hydration rate and achieved maximum swelling at a shorter period which could promote interpenetration of the polymer chain with the tissue. All these factors may have contributed to a higher bioadhesive strength of SCMC films. Residence time on buccal mucosa The mean residence time values of various films on buccal mucosa are depicted in Table 2. As the CP content increased, the duration of adhesion increased proportionally for SCMC films from 16 min for 30% CP to 23 min for 70% CP. However, the increase in duration was not significantly different statistically (p = 0.234). In contrast, the increase in the residence time value for HPMC films was found to be significantly different statistically (p = 0.001). The mean in vivo buccal adhesion time of HPMC films containing 30% CP was significantly lower than 50% and 70% CP, whereas no significant difference was seen between films containing 50% and 70% CP. Hence, 50% CP may deemed to be sufficient as above which no further enhancement in bioadhesive strength was observed. In vitro/in vivo correlation Figure 3 illustrates the relationship between the in vitro bioadhesive strength data and the mean values of in vivo buccal residence time of both the SCMC and HPMC films. Figure 3. Correlation between mean in vitro peak of detachment () and work of adhesion () with mean in vivo residence time. Linear regression lines are shown. The SCMC films exhibited correlation coefficient value of 0.990 for work of adhesion and 0.997 for peak detachment force, while the HPMC films 0.978 for work of adhesion and 0.873 for peak detachment force, respectively. However, when the relationship of these parameters were compared between the two polymers, a greater bioadhesive strength value was obtained in vitro by SCMC films but in vivo by HPMC films, showing absence of rank correlation. Wetting has been reported essential for establishment of intimate contact between the mucoadhesive and mucin/tissue to develop strong adhesive bond (23). Moreover, an increase degree of hydration may increase the chain segment mobility, which could lead to increase interdiffusion of polymer and mucin/tissue (24). In the measurement of in vitro bioadhesive strength, the contact time between the film and tissue wetted with simulated saliva solution was relatively short (10 s). It follows that since SCMC films hydrated at a faster rate and attained maximum swelling at a shorter period, it is thus anticipated that its in vitro bioadhesive strength should be greater than HPMC films which hydrated at a slower rate. However, an optimum water concentration has been reported by Park et al. (25) for hydrocolloid particle to develop maximum adhesive strength and the adhesives would subsequently lose their adhesiveness if they became too susceptible to water since they would be eventually displaced by water. In the evaluation of residence time, the films were placed on the buccal mucosa and hence the contact time was sufficient for both films to reach maximum hydration. As such, SCMC films which achieved maximum swelling faster, would then be expected to be displaced by water sooner than the HPMC films. These may help to explain the higher in vitro bioadhesive strength but shorter in vivo buccal residence time of the SCMC films compared to that of HPMC films. Conclusions In conclusion, CP was found to increase the softness, elasticity and bioadhesive strength of the SCMC and HPMC films. HPMC films appeared to be tougher, more elastic, more bioadhesive in vivo and swell at a more reasonable rate than SCMC films, suggesting that HPMC films may be more preferred as drug vehicle for buccal delivery. Although correlation existed between the in vitro and in vivo bioadhesion data within the polymer, no rank correlation was observed between the two different polymers. References
Corresponding
author: Kok Khiang Peh, School of Pharmaceutical
Sciences,
University of Science Malaysia, 11800 Minden, Penang, Malaysia,
e-mail: kkpeh@usm.my Published by the Canadian Society for Pharmaceutical Sciences. Copyright © 1999 by the Canadian Society for Pharmaceutical Sciences. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||