J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):380-387, 2007
Effect of diltiazem isomers and thiamine on piglet liver microsomal peroxidation using dichlorofluorescein
1Ganesh Rajaraman, 1Guqi Wang, 2Howard J. Smith, 1Yu Gong, and 1,3Frank J. Burczynski
1Faculty of Pharmacy, University of Manitoba, Canada
2Howard J Smith & Associates Pty Ltd, Melbourne, Australia
3Department of Pharmacology, Faculty of Medicine, University of Manitoba, Canada.
Received, June 15, 2007; Revised, July 15 2007; Accepted, August 12, 2007; Published, August 22, 2007.
Corresponding Author: Dr. F.J. Burczynski, Faculty of Pharmacy, University of Manitoba, 50 Sifton Road, Winnipeg, Manitoba, Phone: (204) 474-6902, Email: burczyn@cc.umanitoba.ca
ABSTRACT - PURPOSE. We investigated a potential hepatoprotective role of d-cis diltiazem, l-cis diltiazem, thiamine and the combination d-cis diltiazem and thiamine against lipid peroxidation in a piglet liver microsomal model. A modified in vitro dichlorofluorescein assay was developed to assess the extent of peroxidative damage induced by reactive oxygen species in the piglet liver microsomal fraction. METHODS. Microsomal membrane fraction, obtained from 3 week old female piglets, was treated with either the biologically vasoactive d-cis diltiazem or the non-vasoactive stereoisomer l-cis diltiazem (5-1000 µM) for 1 hour at 37oC followed by one hour incubation with the free radical generator AAPH (2,2'-azobis-(2-amidinopropane) dihydrochloride; 1 mM) to initiate lipid peroxidation. In a separate study, piglet liver microsomes were pre-treated with d-cis diltiazem (50 or 500 µM) and thiamine (10-100 µM) to assess the antioxidant activity of the combination. RESULTS. A dose dependant inhibition of membrane lipid peroxidation was observed with d-cis diltiazem (p<0.05) but not with l-cis diltiazem, suggesting that diltiazem is stereospecific in protecting against microsomal lipid peroxidation. Combining diltiazem with thiamine further protected microsomes against lipid peroxidation compared to use of individual drugs. CONCLUSION. We conclude that diltiazem and the combination of diltiazem and thiamine offers a hepatoprotective effect against free radicals.
Introduction
Calcium channel blockers represent a pharmacologically diverse group of compounds that have been widely used as antihypertensive and antiarrythmic agents (1). These drugs decrease the vascular contractility and arterial tone by modulating the transmembrane influx of calcium through voltage-gated calcium channels (2). d-cis Diltiazem (vasoactive isomer of diltiazem) is a typical calcium channel blocker that is widely used in the treatment of hypertension either alone or in combination with other antihypertensives. Diltiazem also has been demonstrated to protect the liver from warm ischemia (3), carbon tetrachloride hepatotoxicity (4), acetaminophen hepatotoxicity (5), microsomal lipid peroxidation (6), and hepatocellular oxidative stress (7). The hepatoprotective role of diltiazem was initially attributed to its calcium blocking mechanism as most hepatotoxic agents are known to increase intracellular calcium levels. However, diltiazem has been shown to protect rat liver microsomes from lipid peroxidative damage despite the fact that hepatic microsomal preparations lack L-type calcium channels required by the vasoactive d-cis diltiazem for its vascular action (8, 9). This led us to ask the question whether the non-vasoactive l-cis diltiazem (optical isomer of diltiazem) protects hepatic microsomal preparations as well as d-cis diltiazem against lipid peroxidation. If true, l-cis diltiazem could be used as a hepatoprotective agent that is devoid of any vasoactivity (calcium channel blocking) or its associated adverse effects. Thiamine (vitamin B1) is a water-soluble vitamin that plays a crucial role in mammalian carbohydrate metabolism. Despite a great body of literature available on thiamine, little is known about its antioxidant potential against membrane lipid peroxidation. A recent study reported that thiamine protects rat liver microsomes from divalent iron induced lipid peroxidation (10). Therefore, we investigated the antioxidant role of thiamine in piglet liver microsomal lipid peroxidation and whether additional hepatoprotection is offered by the combination of thiamine and diltiazem.
Materials and METHODS
l-cis Diltiazem was purchased from Biomol International (Plymouth Meeting, PA). All other chemicals including d-cis diltiazem, thiamine, and dichlorofluorescein diacetate (DCFH2-DA) were purchased from Sigma Chemical (St. Louis, MO). The aqueous buffer used throughout all experiments was phosphate buffered saline (PBS), which had a composition of (in mM) 137 NaCl, 2.68 KCl, 1.65 KH2PO4, and 8.92 Na2HPO4, pH adjusted to 7.4 using 0.1 N NaOH.
Piglet Liver Microsomes.
All animal studies were performed in accordance with the principles and guidelines of the Canadian Council on Animal Care and the University of Manitoba Fort Garry Campus Protocol Management and Review Committee. Three week old piglets were obtained from the Swine Unit of the University of Manitoba Glenlea Research station. At necropsy, livers were rapidly removed and placed on ice for the preparation of microsomes. Microsomes from hepatocytes were prepared as previously described (11). Briefly, livers were minced with scissors in a homogenizing buffer containing 25 mM Tris, 225 mM sucrose, 5 mM glutathione, and 50 mM sodium fluoride and transferred to a large homogenizer. Liver pieces were homogenized twice at 5000 rpm for 1 min using a Polytron homogenizer (Brinkmann, Westbury, NY) at 4oC with 1 min interval between the two runs. The homogenate was centrifuged at 15,000 x g for 20 min (4oC) followed by centrifugation of the supernatant at 100,000 x g for 1 hr (4oC). The resulting microsomal pellet was washed twice with 0.25 M sucrose and resuspended in 0.25 M sucrose at a concentration of 1 mg/ml. Microsomes were frozen at -80oC in 2 ml aliquots until further use. Liver microsomes obtained from 3 individual piglets were pooled for the purpose of the study.
Lipid Peroxidation Studies.
Piglet liver microsomes (1 mg/ml) were pre-incubated with either l-cis diltiazem or d-cis diltiazem (5, 50, 250, 500, and 1000 µM) or thiamine (10, 50, and 100 µM) for 1 hour at 37oC in a Gyrotory Waterbath Shaker (New Brunswick Scientific, Canada) maintained at 80 rpm (slow shaking). Control microsomes were treated with 0.25 M sucrose. In combined drug studies, microsomes were co-treated with d-cis diltiazem (50 or 500 µM) and thiamine (10, 50, or 100 µM) for 1 hour at 37oC. The microsomes were then incubated for 1 hour with the free radical generator 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH; 1 mM) at 37oC to initiate microsomal lipid peroxidation. At the end of the treatment period, the microsomal mixture was washed and centrifuged several times at 1000 x g using BIOMAX 5000 MWCO ultrafiltration tubes (Millipore, MA) for 10 min with Ca/Mg-PBS to remove excess AAPH, diltiazem, and thiamine. The extent of AAPH-mediated microsomal lipid peroxidation was measured using a modified dichlorofluorescein (DCF) assay procedure.
DCF Assay.
The conversion of the non-fluorescent 2’,7’-dichlorodihydrofluorescein (DCFH) to the highly fluorescent dichlorofluorescein (DCF) by reactive oxygen products, such as lipid peroxides, forms the basis of the assay. The active intracellular 2’,7’-dichlorodihydrofluorescein (DCFH) form of DCFH2-DA was prepared as described by Tollefson et al. (12). Briefly, the diacetate moiety of DCFH2-DA was cleaved by incubating 100 µL stock DCFH2-DA in the presence of 200 µL methanol and 100 µL 2N NaOH at room temperature in the dark. After 2 hours, in the dark, the resulting DCFH solution was diluted with Ca/Mg-PBS to achieve a final probe concentration of 20 µM with the pH adjusted to 7.4 immediately before use.
To measure the extent of lipid peroxidation, 100 µL of the AAPH-treated microsomes was added to 100 µL of the prepared DCFH solution (20 µM) in a 96-well plate at room temperature to give a final DCF concentration of 10 µM. After 15 min of incubation, the fluorescence intensity of emitted DCF fluorescence was measured using a BMG (Durham, NC, USA) Fluostar Galaxy fluorescence plate reader (485 nm excitation wavelength/520 nm emission wavelength). Results were expressed in arbitrary fluorescence units (AFU).
Unbound Diltiazem Concentration:
Piglet liver microsomes (1 mg/ml) were pre-incubated with either l-cis diltiazem or d-cis diltiazem (0, 50, 250, 500 and 1000 µM) for 1 hour at 37°C in water bath and then transferred to microfilters for centrifugation at 2000 rpm for 40 min to separate the unbound from the protein-bound fraction. Analysis of diltiazem was performed as previously described (13). Briefly, an iron (III)–1,10–phenanthroline solution was prepared daily (2 g 1,10-phenanthroline + 0.25 concentrated HCl + FeCl3 + 25ml H2O). The mixture of diltiazem and iron (III)–1,10–phenanthroline solution was heated in a water bath for 40 minutes before spectrophotometric analysis. Standard curve of diltiazem was determined using 0, 10, 40, 160, and 640 µM diltiazem with iron (III)–1,10–phenanthroline solution. Finally, the concentrations of diltiazem in the microsome samples were read using a Spectra Max 190 (Molecular Devices) plate reader at 510 nm.
Statistical Analyses.
Data are presented as mean ± SEM. The n value refers to the number of replicates performed for each study. Data were analyzed using one-way ANOVA with Student-Newman-Keuls post hoc test taking p<0.05 as the level for significance (statistical significance set at p<0.05).
Results
Effect of l-cis diltiazem and d-cis diltiazem on piglet liver microsomes.
Microsomal lipid peroxidation studies were conducted to investigate if diltiazem is stereospecific in protecting piglet liver microsomes from lipid peroxidation. Figure 1 shows DCF fluorescence from peroxidized piglet liver microsomes treated with either l-cis diltiazem or d-cis diltiazem. The vasoactive d-cis diltiazem significantly inhibited AAPH-induced microsomal peroxidation in a dose-dependent manner (P<0.05) compared to control microsomes. The DCF fluorescence was significantly lower even at a low d-cis diltiazem concentration (d-cis diltiazem (0 µM) 53 ± 2; d-cis diltiazem (5 µM): 44 ± 0.5, P<0.05). The emitted fluorescence from the non-vasoactive l-cis diltiazem treated microsomes at the indicated concentrations was not different from control (0 µM) suggesting that l-cis diltiazem does not protect liver microsomes from AAPH-induced lipid peroxidation (Figure 1). Therefore, diltiazem appears to be sterospecific in its protective action against microsomal lipid peroxidation.
Effect of thiamine on piglet liver microsomes.
We furthered our work by investigating the effect of thiamine on hepatic microsomal lipid peroxidation. Results indicated that DCF fluorescence readings from microsomes pre-incubated with thiamine (10 – 100 µM) were significantly lower (P<0.05) compared to control microsomes (no thiamine) and were dose-dependent (Figure 2), suggesting that thiamine protects piglet liver microsomes from AAPH-induced lipid peroxidation.
Effect of d-cis diltiazem and thiamine combination on piglet liver microsomes.
We conducted studies to investigate whether co-treating liver microsomes with d-cis diltiazem and thiamine could further enhance protection against microsomal lipid peroxidation. As shown in Table 1, when microsomes were co-treated with 50 µM d-cis diltiazem and thiamine (10, 50, or 100 µM), DCF fluorescence was significantly reduced compared to 50 µM d-cis diltiazem alone (P<0.05). However, fluorescence values from co-treated microsomes were not different from the respective thiamine concentrations suggesting that combining 50 µM d-cis diltiazem and thiamine did not further enhance the protective effect against microsomal lipid peroxidation compared to thiamine alone. On the other hand, combining 500 µM d-cis diltiazem and thiamine (10, 50, or 100 µM) significantly reduced DCF fluorescence compared to either 500 µM d-cis diltiazem alone or the respective thiamine concentrations (P<0.05). Thus, use of thiamine together with 500 µM d-cis diltiazem significantly enhanced protection against microsomal lipid peroxidation compared to the use of either drug or thiamine alone.
Table 1. DCF fluorescence from AAPH-induced lipid peroxidized hepatic microsomes pre-treated with both thiamine and d-cis diltiazem.
Thiamine (µM) |
Arbitrary Fluorescence Index (AFU) |
||
d-cis diltiazem |
d-cis diltiazem |
d-cis diltiazem |
|
0 |
39260 ± 993 |
36497 ± 235 |
32271 ± 453 |
10 |
31112 ± 414 |
29219 ± 689 * |
28891 ± 457 *∆ |
50 |
27265 ± 736 |
26318 ± 594 * |
23468 ± 590 *∆ |
100 |
23477 ± 448 |
22014 ± 204 * |
21200 ± 180 *∆ |
* Significantly different from the respective d-cis diltiazem concentration (P<0.05). ∆ Significantly different from the respective thiamine concentration (P<0.05). Piglet liver microsomes (1 mg/ml) were pre-incubated with d-cis diltiazem (50 or 500 µM) and thiamine (10, 50, or 100 µM) for 1 hour at 37oC followed by 1 hour incubation with the free radical generator 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH; 1 mM) at 37oC to initiate microsomal lipid peroxidation. Control microsomes were pre-incubated with 0.25 M sucrose. The extent of AAPH-mediated microsomal lipid peroxidation was measured using a modified dichlorofluorescein (DCF) assay procedure. Data represent mean±SEM (n=8).
Unbound Diltiazem Concentration.
Total concentrations of d-cis and l-cis diltiazem used in this study ranged from 0 to 1000 μM. Since diltiazem has limited water solubility it is bound to proteins in the circulation and intracellular proteins within the cell. The pharmacologically effective concentration is thus much lower than that reconstituted. We determined the unbound concentration in our microsome studies to elucidate the concentration that is associated with the antioxidant effect. Figure 3 shows the unbound concentrations of d-cis and l-cis diltiazem in the presence of microsomes. In both cases the unbound concentration and unbound fraction was not statistically different from each other. Overall the unbound fraction was 30.0 ± 5.5% for d-cis diltiazem and 28.1 ± 5.8% for l-cis diltiazem.
Discussion
Calcium antagonists such as diltiazem have been shown to retard the progression of atherosclerosis in various experimental and clinical models (14-16). The antiatherogenic property has been primarily attributed to its vasodilatory effect. Recent experimental evidence, however, suggests that calcium antagonists inhibit membrane lipid peroxidation, an early step in the development of atherosclerosis, by mechanisms independent of its vascular effect (17, 18). Also, a number of calcium antagonists have been shown to inhibit LDL oxidation in vitro and to exhibit antioxidant activity in various membrane preparations (19-21).
Studies with hepatic microsomal fractions have shown that diltiazem protects microsomal membranes from lipid peroxidation by a direct mechanism independent of its calcium flux modulation (8, 9). Diltiazem is thought to modulate physicochemical properties of membrane lipid bilayers by directly interacting with membranes (9). Our finding that d-cis diltiazem confers a dose-dependent protective action against AAPH-induced lipid peroxidation in piglet liver microsomes is in agreement with those studies. A novel finding from the present study is the inability of non-vasoactive l-cis diltiazem to confer a similar effect in hepatic microsomal preparations. This was surprising given the fact that the antioxidant property of diltiazem was thought to be independent of its calcium channel modulation. These results would seem to suggest that the effect of diltiazem is stereospecific in interacting with a specific binding site on membrane lipid bilayers, such as microsomes. To our knowledge, this is the first study to report on the stereospecificity of diltiazem in protecting hepatic microsomal fraction against lipid peroxidation.
l-cis and d-cis diltiazem have been reported to have anti-ischemic action in the isolated, perfused working rat heart suggesting that mechanisms other than calcium channel modulation are responsible for diltiazem’s effect on ischemic myocardium (22). Both diltiazem isomers attenuate hydrogen-peroxide induced derangements in rat hearts partly through an increase in the intracellular myocardial concentration of Na+ (23). Moreover, pre-treatment with l-cis diltiazem reduces the myocardial infarct size in the ischemic-reperfused rabbit heart without affecting any hemodynamic parameters (24). Those studies would seem to suggest a cardioprotective role for l-cis diltiazem in conditions involving oxidative damage. However, l-cis diltiazem failed to protect hepatic microsomal membrane fraction from ROS-mediated lipid peroxidation in the present study. It is not presently clear how the non-vasoactive l-cis diltiazem selectively exhibits cardioprotective properties whereas the vasoactive d-cis diltiazem exhibits both cardioprotective and hepatoprotective properties.
One explanation is that the interacting surface of the hepatic microsomal membrane fraction is stereoselective with respect to diltiazem. For example, d-cis diltiazem could be favored in binding with microsomal membrane fraction over l-cis diltiazem resulting in modulation of membrane physicochemical properties as suggested by Mason et al. (9). Future studies will be required to address these issues and other possibilities.
Another important result from the present studies was that thiamine protected hepatic microsomes from lipid peroxidation. Most of the available literature has focused on the role of thiamine as an essential vitamin (i.e., effects of thiamine deficiency and supplementation) rather than on thiamine as an antioxidant itself. A recent study reported that addition of thiamine (1 – 100 µM) was able to inhibit oxygen-induced lipid peroxidation in mouse liver microsomes and that thiamine oxidation products, thiochrome and thiamine disulfide, were detected on incubating thiamine with oxidized oleic acid (10). Similarly, thiamine was reported to reverse the effects of arsenic-induced oxidative stress (25). This would seem to suggest that thiamine inactivates reactive oxygen species (ROS) by directly interacting with the oxidizing free radicals similar to that of vitamin E. Detailed studies are necessary to systematically investigate the antioxidant nature of thiamine.
As the putative antioxidant action of diltiazem and thiamine are thought to involve different mechanisms, we investigated whether combining diltiazem and thiamine could further enhance protection against lipid peroxidation. Diltiazem is a lipophilic drug and as such could protect membrane lipid bilayers against lipid peroxidation by directly interacting with membrane components. Thiamine, on the other hand, is hydrophilic and as such inactivates ROS by being oxidized within the hydrophilic environment. The combination would be expected to further enhance the protective effects. Our results showed that the combination treatment indeed further protected hepatic microsomes but only using 500 µM d-cis diltiazem concentration (AFU values were at control thiamine levels when 50 µM d-cis diltiazem was used; Table 1). It appears, therefore, that the use of d-cis diltiazem together with thiamine offers further protection compared to use of individual drugs only at a high diltiazem concentration. Whether these observations apply to animal models or clinical scenarios is presently not known and needs to be investigated.
In summary, our work shows that: 1) d-cis diltiazem and not l-cis diltiazem protects hepatic microsomes from lipid peroxidation; 2) thiamine inhibits microsomal lipid peroxidation; and 3) combining thiamine and diltiazem at high doses further enhances protection against lipid peroxidation compared to the use of either compound alone.
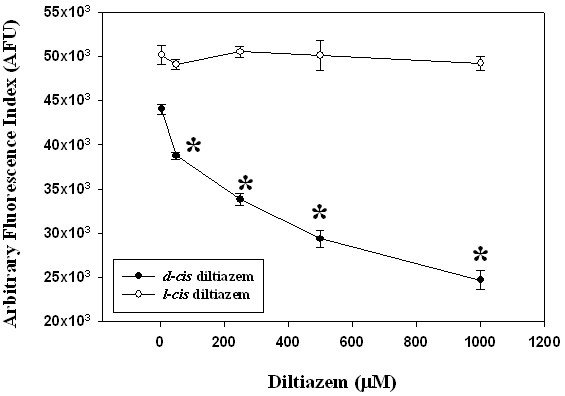
Figure 1. Piglet liver microsomes (1 mg/ml) were pre-incubated with either l-cis diltiazem or d-cis diltiazem (5, 50, 250, 500, and 1000 µM) for 1 hour at 37oC followed by 1 hour incubation with the free radical generator 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH; 1 mM) at 37oC to initiate microsomal lipid peroxidation. Control microsomes were pre-incubated with 0.25 M sucrose. The extent of AAPH-mediated microsomal lipid peroxidation was measured using a modified dichlorofluorescein (DCF) assay procedure. Data represent mean±SEM (n=8). * Significantly different from the control group (P<0.05).
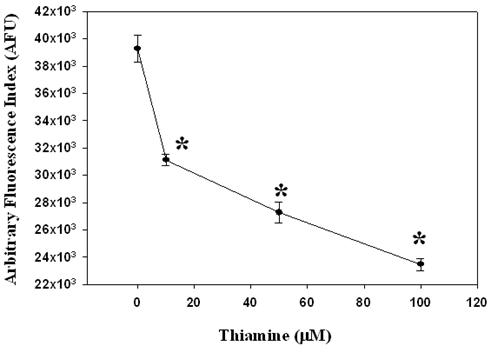
Figure 2. Piglet liver microsomes (1 mg/ml) were pre-incubated with thiamine (10, 50, and 100 µM) for 1 hour at 37oC followed by 1 hour incubation with the free radical generator 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH; 1 mM) at 37oC to initiate microsomal lipid peroxidation. Control microsomes were pre-incubated with 0.25 M sucrose. The extent of AAPH-mediated microsomal lipid peroxidation was measured using a modified dichlorofluorescein (DCF) assay procedure. Data represent mean±SEM (n=6). * Significantly different from the control group (P<0.05).
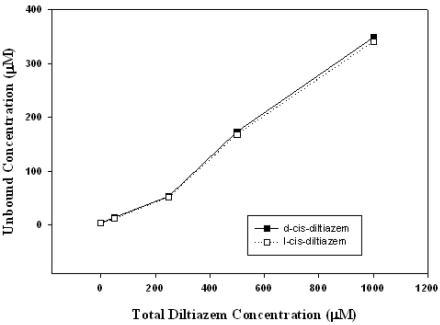
Figure 3. Unbound diltiazem concentration vs total diltiazem concentration in the presence of microsomes. Data represent mean±SEM (n=6).
Acknowledgement
G. Rajaraman gratefully acknowledges support through a University of Manitoba Fellowship Award and the Leslie F Buggey Pharmacy Scholarship. This study was supported by a grant from Howard J Smith & Associates Pty Ltd, Melbourne, Australia and the Canadian Institute of Health Research.
References
| [1] | Bogaert, M.G. How do calcium channel blockers prevent cardiovascular events? Are they all alike? Drugs 52 Suppl 4: 3-7, 1996. |
| [2] | Little, W.C. and Cheng, C.P. Vascular versus myocardial effects of calcium antagonists. Drugs 47 Suppl 4: 41-45, 1994. |
| [3] | Hisanaga, M., Nakajima, Y., Wada, T., Kanehiro, H., Fukuoka, T., Horikawa, M., Yoshimura, A., Kido, K., Taki, J., Aomatsu, Y.,Ueno, M., Ko, S., and Nakano, H. Protective effect of the calcium channel blocker diltiazem on hepatic function following warm ischemia. J Surg Res 55: 404-410, 1993. |
| [4] | Romero, G., Lasheras, B., Sainz Suberviola, L., and Cenarruzabeitia, E. Protective effects of calcium channel blockers in carbon tetrachloride-induced liver toxicity. Life Sci 55: 981-990, 1994. |
| [5] | Satorres, J., Perez-Mateo, M., Mayol, M.J., Esteban, A., and Graells, M.L. Protective effect of diltiazem against acetaminophen hepatotoxicity in mice. Liver 15: 16-19, 1995. |
| [6] | Alov, P., Koleva, M., and Kastelova, A. In vitro effects of calcium channel blockers and beta-adrenergic blocking agents on microsomal lipid peroxidation and cytochrome p-450 content. Exp Toxicol Pathol 51: 277-281, 1999. |
| [7] | Farghali, H., Kmonickova, E., Lotkova, H., and Martinek, J. Evaluation of calcium channel blockers as potential hepatoprotective agents in oxidative stress injury of perfused hepatocytes. Physiological Research / Academia Scientiarum Bohemoslovaca 49: 261-268, 2000. |
| [8] | Koleva, M. and Alov, P. Effect of multiple administration of calcium antagonists on lipid peroxidation in rat liver microsomes. Gen Pharmacol 27: 891-893, 1996. |
| [9] | Mason, R.P., Mak, I.T., Trumbore, M.W., and Mason, P.E. Antioxidant properties of calcium antagonists related to membrane biophysical interactions. The American Journal of Cardiology 84: 16L-22L, 1999. |
| [10] | Lukienko, P.I., Mel'nichenko, N.G., Zverinskii, I.V., and Zabrodskaya, S.V. Antioxidant properties of thiamine. Bulletin of Experimental Biology and Medicine 130: 874-876, 2000. |
| [11] | Lewis, D.S., Oren, S., Wang, X., Moyer, M.L., Beitz, D.C., Knight, T.J., and Mott, G.E. Developmental changes in cholesterol 7alpha-and 27-hydroxylases in the piglet. Journal of Animal Science 78: 943-951, 2000. |
| [12] | Tollefson, K.E., Kroczynski, J., and Cutaia, M.V. Time-dependent interactions of oxidant sensitive fluoroprobes with inhibitors of cellular metabolism. Laboratory Investigation; A Journal of Technical Methods and Pathology 83: 367-375, 2003. |
| [13] | Ayad, M.M., Shalaby, A., Abdellatef, H.E., and Hosny, M.M. New colorimetric methods for the determination of trazodone HCl, famotidine, and diltiazem HCl in their pharmaceutical dosage forms. Analytical and Bioanalytical Chemistry 376: 710-714, 2003. |
| [14] | Ross, R. The pathogenesis of atherosclerosis an update. N Engl J Med 314: 488-500, 1986. |
| [15] | Keogh, A.M. and Schroeder, J.S.. A review of calcium antagonists and atherosclerosis. Journal of Cardiovascular Pharmacology 16 Suppl 6: S28-35, 1990. |
| [16] | Lichtlen, P.R., Hugenholtz, P.G., Rafflenbeul, W., Hecker, H., Jos,t S., and Deckers, J.W. Retardation of angiographic progression of coronary artery disease by nifedipine. Results of the International Nifedipine Trial on Antiatherosclerotic Therapy (INTACT). INTACT Group Investigators. Lancet 335:1109-1113, 1990. |
| [17] | Henry, P.D. Antiperoxidative actions of calcium antagonists and atherogenesis. Journal of Cardiovascular Pharmacology 18 Suppl 1: S6-10, 1991. |
| [18] | Steinberg, D. Antioxidants and atherosclerosis. A current assessment. Circulation 84: 1420-1425, 1991. |
| [19] | Janero, D.R., Burghardt, B., and Lopez, R. Protection of cardiac membrane phospholipid against oxidative injury by calcium antagonists. Biochemical Pharmacology 37: 4197-4203, 1988. |
| [20] | Mak, I.T. and Weglicki, W.B. Comparative antioxidant activities of propranolol, nifedipine, verapamil, and diltiazem against sarcolemmal membrane lipid peroxidation. Circ Res 66: 1449-1452, 1990. |
| [21] | Sobal, G., Menzel, E.J., and Sinzinger, H. Calcium antagonists as inhibitors of in vitro low density lipoprotein oxidation and glycation. Biochemical Pharmacology 61: 373-379, 2001. |
| [22] | Nasa, Y., Ichihara, K., and Abiko, Y. Both d-cis- and l-cis-diltiazem have anti-ischemic action in the isolated, perfused working rat heart. J Pharmacol Exp Ther 255: 680-689, 1990. |
| [23] | Xiao, C.Y., Hara, A., Hashizume, H., Tanaka, K., and Abiko, Y. Both D-cis- and L-cis-diltiazem attenuate hydrogen peroxide induced derangements in rat hearts. European Journal of Pharmacology 374: 387-398, 1999. |
| [24] | Nishida, M., Sakamoto, K., Urushidani, T., and Nagao, T. Treatment with l-cis diltiazem before reperfusion reduces infarct size in the ischemic rabbit heart in vivo. Japanese Journal of Pharmacology 80: 319-325, 1999. |
| [25] | Nandi, D., Patra, R.C., and Swarup, D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology 211: 26-35, 2005. |
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS