J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):368-379, 2007
Predicting Human Intestinal Permeability using Single-pass Intestinal Perfusion in rat
Parvin Zakeri-Milania,b, Hadi Valizadeha,b*, Hosnieh Tajerzadehc, Yadollah Azarmia, Ziba Islambolchilara, Saeed Barzegara, Mohammad Barzegar-Jalalia
a Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
b Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
c Department of Pharmaceutics, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran.
Received, May 5, 2007; Revised August 17, 2007; Accepted, August 20, 2007; Published, August 22, 2007.
Corresponding Author: Dr. Hadi Valizadeh; Faculty of Pharmacy, Tabriz University of Medical Sciences, 51664,Tabriz, Iran; E-mail: valizadeh@tbzmed.ac.ir
ABSTRACT - PURPOSE. The aim of the study was the prediction of human intestinal permeability and fraction absorbed of oral dose using single-pass intestinal perfusion technique (SPIP) in rats. METHODS. Permeability coefficients in anaesthetized rats were determined for 14 compounds. Drug solution in phosphate buffered saline (PBS) was perfused through a ingle-pass intestinal perfusion (SPIP) with flow rate of 0.21 ml/min and samples were taken from outlet tubing at different time points up to 90 min. Phenol red was used as a non-absorbable marker to correct water flux through the segment. Drug concentrations in samples were determined using HPLC and permeability coefficients (Peff) were calculated. RESULTS. The examined compounds demonstrated approximately 12.5 fold difference in magnitude for rat permeability coefficients among themselves. These values were compared with published data for human intestinal permeability, and a strong correlation was found between Peff (rat) and Peff (human); (Peff (human) = 11.04 Peff (rat) – 0.0003; R2= 0.93, P<0.0001). Subsequently the fraction dose absorbed in human (Fa) was estimated and predicted after oral dosing considering Fa(human)=1-e -38450Peff(rat) (R2= 0.91, P<0.0001). CONCLUSIONS. Considering the high correlation of rat Peff values with those of human we conclude that the SPIP could be utilized with precision to predict the human intestinal permeability. It may also be used as a reliable technique to predict the fraction of dose absorbed following oral administration of drug in solution or regular release dosage form in human.
Introduction
Drugs are most commonly administered via oral route. In fact the vast majority of pharmaceutical dosage forms are designed for oral administration. However not all of the compounds have compatible properties for the development of oral dosage forms. Often poor bioavailability result in the termination of development of new drugs. Therefore, optimized bioavailability of drugs is one of the most important goals for the pharmaceutical industry.
The two principal routes of absorption across small intestinal epithelium are paracellular and transcellular. Typically, lipophilic drugs are absorbed by the transcellular route, whereas hydrophilic drugs are slowly absorbed via the transcellular pathway or in some cases via the paracellular route. Both passive and active (carrier-mediated) processes may contribute to the permeability to drugs transported by the transcellular pathway (1). For instance several amino acid analogues such as α-methyldopa (2), L-dopa (3) and baclofen (4) are transported by large neutral amino acid transporter and orally absorbed cephalosporins are substrates for the H+/oligopeptide transporter (5). The efficiency of drug absorption is also influenced by efflux proteins lining the small intestine. For example P-glycoprotein (P-gp) is a phosphorylated and glycosylated efflux protein belonging to a family of plasma membrane proteins encoded by the multidrug resistance gene(s) (1). It functions as a membrane-localized drug transport mechanism that has the ability to actively pump its substrates out of the cell. This could reduce the efficiency of absorption of common, orally absorbed drugs like digoxin, carbamazepine and propranolol. Recognition of the much broader specificity of P-gp and its functional effects on intestinal drug transport could lead to strategies for improving absorption, either by incorporating structural features in drug design that reduce interaction with P-gp or by the use of specific P-gp inhibitors (1).
Bioavailability represents the rate and extent of oral dose reaching the blood circulation which is controlled by (i) solubility and dissolution rate of a drug in the intestinal fluid and (ii) permeability across the intestinal membrane (Peff), pre-systemic metabolism and, sometimes, the efficiency of drug transporting system. In this paper we have concentrated on the permeability issue only. It is clear that the accurate intestinal permeability for drugs and nutrients is difficult to directly study in human. It is also unrealistic to predict the ability of a drug molecule to cross the intestinal barrier from simple physicochemical measurements such as pKa, molecular size and partition coefficient. Therefore a number of in vitro and in situ experimental models have been developed which determine the intestinal absorptive potential of a drug and the mechanism of absorption (6-11) . Among these methods single-pass intestinal perfusion (SPIP) approach is the most frequently used technique which provides conditions closer to what is faced following oral administration (12, 13). SPIP technique possesses a preserved microclimate above the intestinal membrane which makes it less sensitive to pH variations (14). This technique provides the unique advantages of experimental control (compound concentration and intestinal perfusion rate) and the ability to study regional differences; factors that may influence the intestinal absorption of a compound. The basic experimental procedure has been described by Fagerholm et al (15) in which the compound of interest is monitored in perfusate and loss of compound (difference between inlet and outlet concentrations) is attributed to permeability after preliminary studies including stability studies in the perfusate medium and adsorption studies with the syringe and tubing used in the experiments. Since water absorption and secretion during the perfusion may cause errors in the calculated Peff values, a non-absorbable marker to correct water flux is needed (13). For this purpose phenol red is co-perfused with drug compounds. It was first introduced as a non-absorbable marker by Gorham in 1923 (16) . Previous studies have shown that the extent of absorption in humans can be predicted from single-pass intestinal perfusion technique in rat (11, 15), however, in this study we compare the quantitative differences between permeabilities in human and rat models directly using a larger number of model drugs with a broad range of physicochemical properties for both high and low permeability classes of drugs. In fact more poorly absorbed drugs (cimetidine and ranitidine) have been included in the present work and therefore it is likely that the obtained equations will give a more reliable prediction of the human intestinal permeability and fraction of dose absorbed than previously reported equations. Two actively transported drugs (cephalexin and α-methyl dopa) have been added to the list because they are absorbed by active transport mechanisms. This was to account for this factor in the relationship between permeability values in rat and human.
Moreover the rat in situ intestinal permeability values for tested compounds are presented which some of them have not yet been reported. Human intestinal permeabilities have been obtained from published data in which the regional perfusion approach in human jejunum was used. The goal was to obtain a more reliable correlation to predict human intestinal permeability and fraction of dose absorbed using rat intestinal permeability. The obtained values were compared with previously published data in the rat as well.
Materials and METHODS
Chemicals:
Naproxen and ketoprofen were provided from Sigma (St. Louis, MO, USA) and Wako (Osaka, Japan) respectively. Furosemide and Hydrochlorothiazide were purchased from Shasun Chemicals and Drugs LTD. (Pondicherry, India). Propranolol was obtained from ICI-Pharma (Madrid, Spain) and metoprolol was from Ciba-Geigy (Barcelona, Spain). Phenol red was purchased from Sigma chemical company (St. Louis, MO, USA). Acetonitrile and methanol were HPLC grade and obtained from Merck (Darmstadt, Germany). KH2PO4, NaH2PO4, Na2HPO4, Orthophosphoric acid, NaOH, NaCl, glacial acetic acid and triethylamine were purchased from Merck (Darmstadt, Germany) as well. Double distilled water was used during the entire HPLC procedure.
Single-pass perfusion of the rat jejunum:
Single-pass intestinal perfusion studies in rats were performed using established methods adapted from the literature (17, 18). Briefly, male Wistar rats (weight, 200-250 g; age, 7-9 weeks) were maintained on 12 h light- dark cycle and fasted 12-18 h before experiment with free access to water. Rats were anaesthetized using an intraperitoneal injection of pentobarbital (60 mg/kg) and placed on a heated pad to keep normal body temperature. The small intestine was surgically exposed and 10 cm of jejunum was ligated for perfusion and cannulated with plastic tubing (0.04 in. i.d., 0.085 in. o.d.). The cannulated segment rinsed with saline (37oC) and attached to the perfusion assembly which consisted of a syringe pump (Palmer, Surrey, UK) and a 60 ml syringe was connected to it. Care was taken to handle the small intestine gently and to minimize the surgery in order to maintain an intact blood supply. Blank perfusion buffer was infused for 10 min by a syringe pump followed by perfusion of compounds at a flow rate of 0.2 ml/min for 90 min. The perfusate was collected every 10 min in microtubes. The length of segment was measured following the last collection and finally the animal was euthanitized with a cardiac injection of saturated solution of KCl. Samples were frozen immediately and stored at -20oC until analysis. In all animal studies “Guide to the care and use of experimental animals” by Canadian Council on Animal Care, was followed (19).
Composition of perfusion solution:
The perfusion buffer composition was as follows (mM): 40 Na2HPO4 (anhydrous), 26 NaH2PO4.2H2O and 119 NaCl. Phenol red (0.7mM) was added to the solution as a non-absorbable marker in each experiment. The inlet perfusate concentration (Cin) and molecular weight of each compound used in SPIP studies are shown in Table 1. The pH of prepared buffer was adjusted to 7.2. Preliminary experiments revealed that no considerable adsorption of the compounds on the tubing and syringe took place.
Stability Tests:
The stability of compounds was tested by their incubation in the perfusion solution and blank perfusate from rat intestine at 37±1° C for 2 h. Samples were taken at 0, 1, and 2 h post-perfusion. Then the samples were analyzed by HPLC. The blank perfusate was obtained by passing the blank perfusion buffer through a segment of intestine in situ at a flow rate of 0.2 ml/min. There was no sign of degradation of compounds during this period of time. However, methyl dopa seemed labile, hence, its solutions were protected from light.
Analytical methods:
All samples were analyzed by reverse-phase high performance liquid chromatography using Shimpack VP-ODS 5 mm 4.6 x 250 mm with a Shimpack VP-ODS 5 mm 4.6 x 50 mm guard column. The chromatographic conditions for all tested compounds are listed in table 1. The mobile phases were filtered through sintered glass filter P5 (1-1.6 micron) (Winteg, Germany) and degassed in sonicator (Liarre, Italy) under vacuum and then were pumped in isocratic mode in all cases.
Data analysis:
Effective permeability coefficients (Peff) were calculated from the steady-state concentrations of compounds in the collected perfusate which is considered to be reached when the concentration level of phenol red was at the steady state level. It was reached about 40 min after the beginning of the perfusion which is confirmed by plotting the ratio of the outlet to inlet concentrations (corrected for water transport) versus time. A plot of steady-state concentration in collected samples is shown in Fig. 1 for ranitidine as a sample.
The intestinal net water flux (NWF, μl/h/cm) was calculated according to Eq. (1):

Where [Ph.red (in)] and [Ph.red (out)] are the inlet and outlet concentrations of the non-absorbable, water flux marker phenol red. A negative net water flux indicates loss of fluid from the mucosal side (lumen) to the serosal side (blood). A positive net water flux indicates secretion of fluid into the segment (16).
Peff was calculated using following equation (Eq.2) according to the parallel tube model (29, 30):
Eq.(2)
In which Cin is the inlet concentration and Cout is the outlet concentration of compound which is corrected for volume change in segment using phenol red concentration in inlet and outlet tubing. Q is the flow rate (0.2 ml/min), r is the rat intestinal radius (0.18 cm)(29) and l is the length of the segment. It has been demonstrated that in humans at a Qin of 2-3 ml/min, Peff is membrane-controlled. In the rat model the Qin is scaled to 0.2 ml/min, since the radius of the rat intestine is about 10 times less than that of human (15).
The spearman rank correlation coefficient which can be used to summarize the strength and direction (negative or positive) of a relationship between two set of observations was calculated using the Eq.(3):
rs= 1- (6Σd2)/(n3-n)
Eq.(3)
Where d is the difference of the two ranks and n is the number of compounds in the experiment (31).
Table 1. Chromatographic conditions for analysis of tested drugs
Compound |
Limit of quantitation |
mobile phase |
Wave length (nm) |
Naproxen (20) |
0.25 |
19.9 %(v/v) methanol, 27.9 %(v/v) acetonitrile, 51.8 % (v/v) water and 0.4 %(v/v) triethylamine (adjusted to pH 3.2) |
280 |
Ketoprofen (20) |
0.3 |
||
Furosemide (21) |
0.7 |
42% (v/v) acetonitrile, 58% (v/v) water, 0.9 % (v/v) glacial acetic acid and 0.1% (v/v) triethylamine (adjusted to pH 5.6) |
270 |
Antipyrine (21) |
100 |
||
Hydrochlorothiazide (21) |
0.9 |
||
Metoprolol (22) |
14 |
55% (v/v) methanol, 45%(v/v) KH2PO40.05 M (adjusted to pH 6) and 0.2 %(v/v) triethylamine |
227 |
Propranolol (22) |
7.2 |
||
Piroxicam (23) |
60 |
39 % (v/v) acetonitrile, 61% (v/v) sodium acetate 0.1 M and 0.05% (v/v) triethylamine (adjusted to pH 2.6) |
330 |
Atenolol (24) |
195 |
10%(v/v) acetonitrile, 90%(v/v) phosphate buffer 0.67 M(pH=7.4) and 0.2%(v/v) triethylamine (adjusted to pH 3) |
225 |
Cimetidine |
28 |
78%(v/v) KH2PO4 0.05 M, 22%(v/v) acetonitrile and 0.05% (v/v) triethylamine (adjusted to pH 8) |
229 |
Ranitidine |
42 |
||
Carbamazepine (25) |
15 |
67%(v/v) methanol, 33% (v/v) water and 1% glacial acetic acid |
230 |
Phenol red (26) |
100 |
45%(v/v) KH2PO4 0.05 M and 55% (v/v) methanol (adjusted to pH 2.6) |
430 |
Cephalexin (27) |
700 |
81% (v/v) NaH2PO4 0.1 M and 0.19%(v/v) methanol |
254 |
Ibuprofen (28) |
24 |
85% (v/v) acetonitrile, 15% (v/v) phosphate buffer 0.067 M and 0.2%(v/v) Orthophosphoric acid |
254 |
α -Methyl dopa |
19 |
73%(v/v) KH2PO4 0.05 M, 27%(v/v) acetonitrile and 0.05% (v/v) triethylamine (adjusted to pH 6) |
279 |
CV<8% for all assays. |
|||
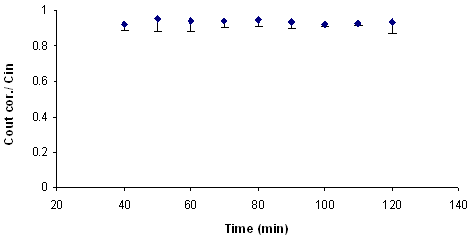
Figure 1. Representative plot of the concentration ratio of the outlet and inlet concentrations vs. time for ranitidine in single-pass intestinal-perfusion in rat. (Error bars represent S.D.)
Results
In any in situ intestinal perfusion technique it is necessary to determine the extent of volume changes of the solution in the gut lumen during an experiment. For this purpose phenol red dye (0.7 mM) was added to drug solution in each experiment. Phenol red was used as a non-absorbable marker to detect gain or loss of water by the lumen. This factor did not appear to influence the absorption of compounds used in this study. The stable water fluxes and permeability coefficients in each perfusion, as a function of time, for compounds transported passively and by a carrier-mediated mechanism indicated that intestinal barrier function was maintained during the procedure. The determined Peff and NWF values for tested compounds in the single pass intestinal perfusion technique are listed in Table 2.
Discussion
According to Yuasa et al, anesthesia influences the intestinal absorption in rats. In fact surgery and anesthesia may cause reduction in blood flow and motility which cause decreases of both passive and active transport. Anaesthetics may also directly affect the cell membranes (32). It has been reported that barbiturates have the least effect on intestinal permeability in rats (32); therefore we have used pentobarbital (60 mg/kg) as an anaesthetic agent in all experiments. In addition, as an important aspect, the potential age-dependency of rat intestinal permeability should be considered. Although an age-dependent intestinal permeability might be valid for very young and very old rats, no influence of age on the jejunal permeability in the rat within the age interval of 5-30 weeks has been reported (33). In the present study, compounds with different physicochemical properties and reported Peff values in human intestine were chosen. In 1998 Chiou and Barve (34) reported a great similarity in oral absorption (Fa) between rat and human; however they have used an in vivo method, quite different from in situ techniques, that can give an idea of the absorption from the entire GI tract, therefore the significance of rat jejunal permeability values for predicting the human Fa has not been tested in that report. In the present study the obtained Peff values ranged between 2 ×10-4 cm/sec to 1. 6 ×10-5 cm/sec and showed a high correlation (R2=0.93, P<0.0001) with human Peff data for passively absorbed compounds (Fig 2) confirming the validity of our procedure.
The human absorption parameters are presented in Table 3. This correlation was weakened when the actively transported compounds (cephalexin and α methyl dopa) were added to the regression (R2=0.87, P<0.0001). In Fig.3 the plot of predicted vs observed human Peff values is shown which presents a high linear correlation with intercept not markedly different from zero (R2= 0.93, P <0.0001).
Table 2. The molecular weights, inlet concentration, mean water fluxes and mean Peff values of tested compounds
Compound |
Molecular weight |
Cin |
Water flux (μl/min/cm) |
Mean Peff |
Antipyrine |
188.23 |
1.06 |
1.4 ± 0.2 |
5.9 ± 0.2 |
Propranolol |
259.3 |
0.135 |
-1.9 ± 3.4 |
5.6 ± 2.0 |
Carbamazepine |
236.27 |
0.42 |
3.4 ± 2.6 |
6.2 ± 0.6 |
Ibuprofen |
206.28 |
1.93 |
1.8 ± 4.5 |
20 ± 2.2 |
Ketoprofen |
254.28 |
0.19 |
1.1 ± 0.8 |
9.6 ± 1.8 |
Naproxen |
230.26 |
0.99 |
0.9 ± 0.8 |
1.1 ± 0.2 |
Piroxicam |
331.35 |
0.03 |
4.3 ± 1.5 |
7.9 ± 4.0 |
Metoprolol |
267.36 |
0.07 |
-1.9 ± 3.4 |
3.3 ± 1.5 |
Furosemide |
330.75 |
0.12 |
0.1 ± 1.7 |
3.3 ± 2.0 |
Cimetidine |
252.34 |
0.39 |
0.3 ± 2.3 |
4.8 ± 0.1 |
Atenolol |
266.34 |
0.37 |
-0.0 ± 0.8 |
1.6 ± 0.02 |
Ranitidine |
314.37 |
0.31 |
0.6 ± 1.5 |
2.2 ± 1.0 |
Hydrochlorothiazide |
297.74 |
0.16 |
0.0 ± 1.9 |
2.0 ± 1.0 |
Cephalexin |
347.39 |
0.28 |
0.6 ± 0.5 |
6.4 ± 3.6 |
α-methyl dopa |
211.21 |
0.47 |
-0.06 ± 0.85 |
2.3 ± 0.73 |
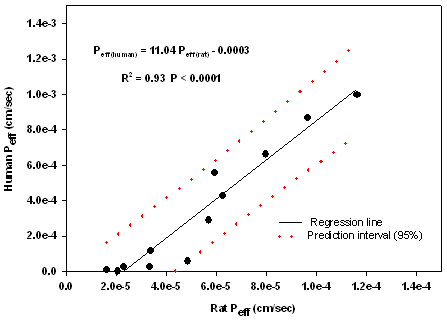
Figure 2. Plot of Peff rat vs Peff human.
Table 3. The human intestinal permeability values and fraction absorbed (%) for model drugs
Compound |
human Peff (10-4) (cm/sec) |
human Fa |
Atenolol |
0.12a |
0.50d |
Ranitidine |
0.27b |
0.50d |
Hydrochlorothiazide |
0.04b |
0.54b |
Furosemide |
0.30c |
0.61d |
Metoprolol |
1.20a |
0.95d |
Cimetidine |
0.60b |
0.79e |
Propranolol |
2.90b |
0.90d |
Antipyrine |
5.60b |
1.00d |
Carbamazepine |
4.30b |
0.97g |
Piroxicam |
6.65b |
0.99f |
Ketoprofen |
8.70b |
1.00c |
Naproxen |
10.0a |
1.00f |
Cephalexin |
1.56b |
0.98d |
α methyl dopa |
0.10b |
0.45f |
a taken from ref. (35) b taken from ref. (36) c taken from ref. (24) d taken from ref. (37 ) e taken from ref. (38) f taken from ref. (39) g taken from ref. (40).
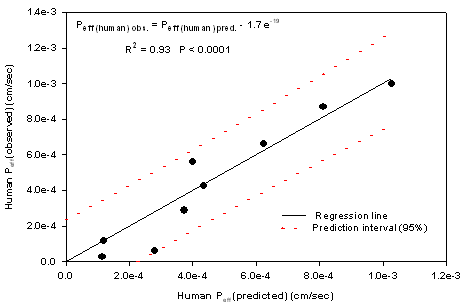
Figure 3. Plot of predicted human Peff vs observed human Peff based on SPIP model.
According to previously reported equations by Salphati et al (11) in the ileum and Fagerholm et al (15) in the jejunal segment, the slopes for the same correlation between two models were 6.2 and 3.6 respectively. However based on our results for larger set of compounds including more low-permeable drugs the rat Peff values were on average 11 times lower than those in human. The species differences and the differences in effective absorptive area might be the reasons for the lower permeability values in the rat model. In addition, any changes in the intestinal barrier function during the surgery might be a main reason for obtaining different results in literature concerning intestinal permeability of drugs.
A strong correlation was observed between rat permeability data and fraction of oral dose absorbed in human fitting to chapman type equation; Fa(human)= 1- e -38450Peff(rat) (R2= 0.91, P<0.0001) (Fig. 4). The same fitting using human intestinal permeability gives a lower correlation coefficient which is presented in Fig.5.
The comparison of rat Peff and intestinal absorption in man (Fa) showed that rat Peff values greater than 5.9×10-5 cm/sec corresponds to Fa ≈ 1 while rat Peff values smaller than 3.32×10-5 cm/sec corresponds to Fa values lower than 0.6. Corresponding estimates in human are > 0.2×10-4 cm/sec and <0.03×10-4 cm/sec, respectively. Moreover the predicted and observed human Fa (%) are linearly correlated (R2 = 0.92, P <0.0001) (Fig.6).
The rank order for Peff values in rat was compared with those of human Peff and Fa. The spearman rank correlation coefficients (rs) were found to be 0.96 and 0.91 respectively (Table 4).
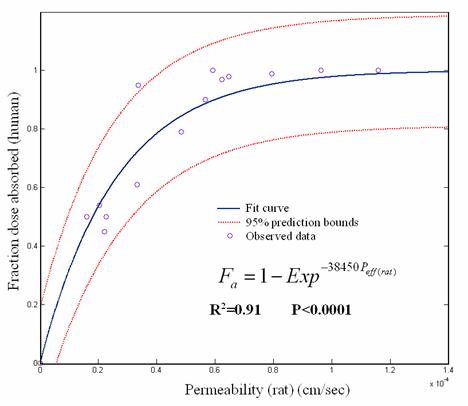
Figure 4. Plot of rat Peff vs human Fa
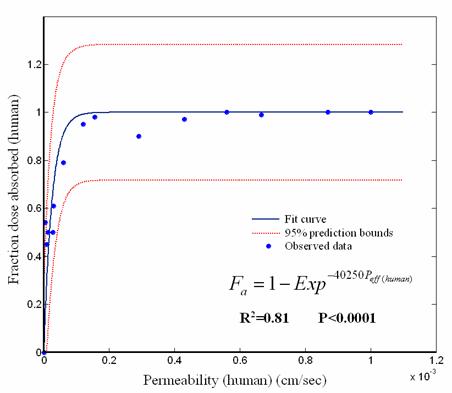
Figure 5. Plot of human intestinal permeability values vs human Fa.
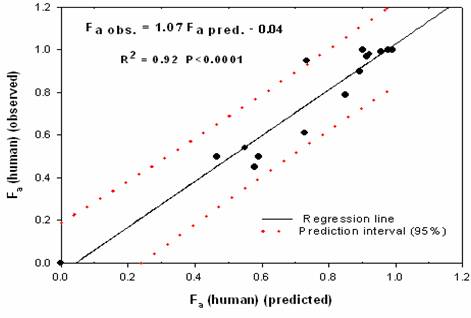
Figure 6. Plot of predicted human Fa vs observed human Fa based on SPIP model.
Table 4. The rank order of rat and human Peff values and human Fa for tested compounds in rat perfusion technique.
Compound |
Rank order |
||
Rat Peff |
Human Peff |
Human Fa |
|
Atenolol |
13 |
11 |
12.5 |
Ranitidine |
12 |
10 |
12.5 |
Hydrochlorothiazide |
11 |
12 |
11 |
Furosemide |
10 |
9 |
10 |
Metoprolol |
9 |
7 |
7 |
cimetidine |
8 |
8 |
9 |
Propranolol |
7 |
6 |
8 |
Antipyrine |
6 |
4 |
2 |
Carbamazepine |
5 |
5 |
6 |
Piroxicam |
4 |
3 |
4.5 |
Ketoprofen |
3 |
2 |
2 |
Naproxen |
2 |
1 |
2 |
Ibuprofen |
1 |
- |
4.5 |
Rat perfusion vs human intestinal permeability * rs = 0.96 (n=12) |
|||
Rat perfusion vs absorption in man * rs = 0.91 (n=13) |
|||
Based on the obtained results, we conclude that in situ perfusion technique in rat may be used as a reliable technique to predict human gastrointestinal absorption extent following oral administration of a drug. However, to render our observation more reliable, it seems that using larger number of compounds belonging to all four biopharmaceutical classes, i.e., different solubility and permeability properties (41) especially drugs with low permeability must be tested.
CONCLUSION
The rat and human jejunal Peff values are highly correlated for passively absorbed compounds, therefore, both can be used with precision to predict in vivo oral absorption in man. This is not unexpected because the SPIP technique provides an intact blood supply and a functional intestinal barrier, conditions very close to normal physiological state.
References
| [1] | D. R. Friend, Drug Delivery to the Small Intestine, Current Gastroenterology Reports, 6: 371-376, 2004. |
| [2] | G. L. Amidon, A. E. Merfeld, J. B. Dressman. Concentration and pH dependency of a-methyldopa absorption in rat intestine. J Pharm Pharmacol, 38: 363-368, 1986. |
| [3] | H. Shindo, T. Komai, K. Kawai. Studies on the metabolism of D- and L-isomers of 3, 4-dihydroxyphenylalanine (DOPA). V. Mechanism of intestinal absorption of D- and L-DOPA-14C in rats. Chem Pharm Bull (Tokyo), 21: 2031-2038, 1973. |
| [4] | T. Cercos-Fortea, A. Polache, A. Nacher, E. Cejudo-Ferragud, V. G. Casabo, M. Merino. Influence of leucine on intestinal baclofen absorption as a model compound of neutral α-amino acids. Biopharm Drug Dispos, 16: 563-577, 1995. |
| [5] | A. Tsuji. Intestinal absorption of β-lactam antibiotics; In M. D. Taylor and G. L. Amidon (eds.), Peptide-based drug design, American Chemical Society, Washington, DC, pp. 299-316, 1995. |
| [6] | G.L. Amidon, P.J. Sinko, D. Fleisher. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res, 5:651-654, 1988. |
| [7] | P. Artursson, J. Karlsson. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial cells. Biochem Biophys Res Commun, 175:880-885, 1991. |
| [8] | T.J. Cook, S.S. Shenoy. Intestinal permeability of chlorpyrifos using a single-pass intestinal perfusion method in the rat. Toxicology, 184:125-133, 2003. |
| [9] | K.M. Hillgren, A.Kato, R.T Borechard. In vitro systems for studying intestinal drug absorption. Med Res Rev, 15:83-109, 1995. |
| [10] | W. Rubas, N. Jezyk, G.M. Grass. Comparison of the permeability characteristics of a human colonic epithelial (Caco-2) cell line to colon of rabit, monkey and dog intestine and human drug absorption. Pharm Res, 10:113-118, 1993. |
| [11] | L. Salphati, K. Childers, L. Pan, K. Tsutsui, L. Takahashi. Evaluation of a single-pass intestinal-perfusion method in rat for the prediction of absorption in man. J Pharm Pharmacol, 53:1007-1013, 2001. |
| [12] | W. Crouthamel, A.C.E. Sarapu. Animal models for oral drug delivery in man. American Pharm Assoc, Washington DC, p. 27,1983. |
| [13] | S.C. Sutton, M.T.S. Rinaldi, K.E. Vukovinsky. Comparison of the Gravimetric, Phenol Red, and 14C-PEG-3350 Methods to Determine Water Absorption in the Rat Single-Pass Intestinal Perfusion Model. AAPS PharmSci (http://www.pharmsci.org), 3, 2001. |
| [14] | M.L.Hogerle, D.Winne. Drug absorption by the rat jejunum in situ. Dissociation from the ph-partition theory and role of microclimate pH and unstirred layer. Naunyn Schmiedebergs Arch Pharmacol, 322:249-255, 1983. |
| [15] | U. Fagerholm, M. Johansson, H. Lennernas. Comparison between permeability coefficients in rat and human jejunum. Pharm Res, 13:1336-1342, 1996. |
| [16] | F.D. Gorham. The factor of dilution in gastric analysis. J Amer Med Ass, 81:1735-1742, 1923. |
| [17] | U.S.H. Sevensson. High in situ rat intestinal permeability of artemisinin unaffected by multiple dosing and with no evidence of P-gp involvement. Drug Metab Dispos, 27:227-232, 1999. |
| [18] | P.J. Sinko, P. Hu, A.P. Waclawski, N.R. Patel. Oral absorption of anti-AIDS nucleoside analogues. 1. Intestinal transport of didanosine in rat and rabbit preparations. J Pharm Sci, 84:959-965, 1995. |
| [19] | D. Ernest, E.D. Alfert, M. Brenda, B.M. Cross, A.A.E. Mcwilliam. CCAC, Guide to the care and use of experimental animals, Canadian Council on Animal Care. vol1, 1993. |
| [20] | P. Zakeri.-Milani., M. Barzegar-Jalali, H. Tajerzadeh, Y. Azarmi, H. Valizadeh. Simultaneous determination of naproxen, ketoprofen and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal, 39:624-630, 2005. |
| [21] | H. Valizadeh , P. Zakeri-Milani, Z. Islambulchilar, H. Tajerzadeh. A simple and rapid HPLC method for determining Furosemide, Hydrochlorothiazide and Phenol red: Applicability to Intestinal permeability studies. J AOAC Int, 89:1-6, 2005. |
| [22] | P. Zakeri-Milani, H. Valizadeh, Y. Azarmi, M.Barzegar-Jalali, H. Tajerzadeh. Simultaneous determination of metoprolol, propranolol and phenol red in samples from rat in situ intestinal perfusion studies, Daru. 14:102-108, 2006. |
| [23] | S. Dadashzadeh, A.M. Vali, N. Rezagholi. LC determination of Piroxicam in human plasma. J Pharm Biomed Anal, 28:1201-4, 2002. |
| [24] | P. Modamio, C.F. Lastra, J. Maritto. Error structure for the HPLC analysis for atenolol, metoprolol and propranolol: a useful weighting method in parameter estimation. J Pharm Biomed Anal, 17:507-13, 1998. |
| [25] | L.E. Raid, R.J. Sawchuk. Simultaneous determination of carbamazepine and its epoxide and transdiol metabolites in plasma by microbore liquid chromatography. Clin Chem, 37:1863-1866, 1988. |
| [26] | E. S. Swenson, B. William, B. Malison, W Curatolo. Intestinal permeability enhancement: Efficacy, Acute local toxicity and reversibility. Pharm Res, 11:1132-42, 1994. |
| [27] | P.G. Welling, A. Selen, J.G. Pearson, F. Kwok, M.C. Rogge, A. Ifan, D. Marrero, W.A. Craig, C.A. Johnson. A pharmacokinetic comparison of cephalexin and cefadroxil using HPLC assay procedures. J Biopharm Drug Dispos, 6:147-57, 1985. |
| [28] | C.A. Phillips, B.B. Michniak. Transdermal delivery of drugs with differing lipophilicities using azone analogs as dermal penetration enhancers. J Pharm Sci, 84:1427-1433, 1995. |
| [29] | I. Komiya, J.Y. Park, A. Kamani, N.F.H. Ho, W.I. Higuchi. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int J Pharm, 4:249-262, 1980. |
| [30] | M.D. Levitt, J.M.Kneip, D.G. Levitt. Use of laminar flow and unstirred layer models to predict intestinal absorption in the rat. J. Clin. Invest., 81:1365-1369, 1988. |
| [31] | L.D. Fisher, G. Van Belle. Association and prediction: Linear models with one predictor variable. In: Biostatistics, a methodology for the health science, Wiley-Interscience, new York, pp. 345-417, 1993. |
| [32] | H. Yuasa, K. Matsuda, J. Watanabe. Influence of anaesthetic regimens on intestinal absorption in rats. Pharm Res, 10:884-888, 1993. |
| [33] | A. Lindahl, E. Krondhal, A. Gruden, A. Ungell, H. Lennernas. Is the jejunal permeability in rats age-dependent? Pharm Res, 14:1278-1281, 1997. |
| [34] | W.L. Chiou, A. Barve. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharm Res, 15:1792-1795, 1998. |
| [35] | H. Lennernas. Human intestinal permeability. J Pharm Sci, 87:403-410,1998. |
| [36] | N.A. Kasim, , M. Whitehouse, , C. Ramachandran, , M. Bermejo, H.Lennernas, A.S. Hussain, H.E. Junginger, S.A. Stavchansky, K.K. Midha, V.P. Shah, G.L. Amidon. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Molecular Pharmaceutics, 1:85-96, 2003. |
| [37] | J.D. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J.W. Tolan, H.E. Selick, J.R. Grove. MDCK cells: A tool for membrane permeability screening. J Pharm Sci, 88:28-33, 1998. |
| [38] | P. Stenberg, U. Norinder, K. Luthman, P. Artursson. Experimental and computational screening models for the prediction of intestinal drug absorption. J Med Chem, 44:1927-1037, 2001. |
| [39] | E. Walker, S. Janich, B.J. Roessler, J.M. Hilfinger, G.L. Amidon. HT29-MTX/Caco-2 cocultures as an in vitro model for the intestinal epithelium: In vitro- in vivo correlation with permeability data from rats and humans. J Pharm Sci, 85:1070-1076, 1996. |
| [40] | J.B. Dressman, G.L. Amidon, D. Fleisher. Absorption potential: estimating the fraction absorbed for orally administered compounds. J Pharm Sci, 74:588-589, 1985. |
| [41] | R. Loebenberg and G.L. Amidon, Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm, 50(1): 3-12, 2000. |
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.
CSPS Home | JPPS Home | Search | Subscribe to JPPS