J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):332-339, 2007
Effect of medium pH on the cytotoxicity of hydrophilic statins
Masaki Kobayashi, Toshiki Kagawa, Rumi Takano, Shirou Itagaki, Takeshi Hirano, Ken Iseki
Laboratory of Clinical Pharmaceutics & Therapeutics, Division of Pharmasciences, Faculty of Pharmaceutical Sciences, Hokkaido University, Kita-12-jo, Nishi-6-chome, Kita-ku, Sapporo 060-0812, Japan
Received, May 3, 2007; Revised, June 10, 2007; Accepted June 25, 2007; Published June 25, 2007.
Corresponding Author: Ken Iseki, Ph. D., Laboratory of Clinical Pharmaceutics & Therapeutics, Division of Pharmasciences, Faculty of Pharmaceutical Sciences, Hokkaido University Kita-12-jo, Kita-ku, Sapporo 060-0812, Japan e-mail: ken-i@pharm.hokudai.ac.jp
ABSTRACT - PURPOSE. The aim of this study was to examine the mechanism of pravastatin- and rosuvastatin-induced cytotoxicity and the relationship between pravastatin- and rosuvastatin-induced cytotoxicity and medium pH using human prototypic embryonal rhabdomyosarcoma cell line (RD) and rat myoblast cell line (L6) as a model of in vitro skeletal muscle. METHODS. Statin-induced reduction of cell viability and apoptosis was measured by 3-(4,5-dimethylthiazol-2-yl)2,5 -diphenyl tetrazolium bromide (MTT) assay and caspase assay. Intracellular accumulation of statins was determined using an HPLC system. RESULTS. Rosuvastatin cytotoxicity, reduction of cell viability, morphological changes and caspase activation at acidic pH (pH 6.8) were significantly greater than those at neutral pH (pH 7.4). Rosuvastatin accumulation at acidic pH was greater than that at pH 7.4. On the other hand, medium pH had no effect on pravastatin accumulation. CONCLUSIONS. Rosuvastatin cytotoxicity at acidic pH is associated with increasing intracellular accumulation of rosuvastatin. On the other hand, medium pH had no effect on cytotoxicity of pravastatin.
Introduction
3-Hydroxy-3-methylglutaryl coenzyme A (HMG -CoA) reductase inhibitors such as statins are the most widely used cholesterol-lowering agents for prevention of obstructive cardiovascular events [1-3]. However, one of the most important clinical adverse effects in therapy with statins is drug-induced skeletal muscle toxicity (rhabdomyolysis) [4]. Skeletal muscle abnormalities can range from benign myalgia to myopathy, which is defined as a ten-fold elevation of the creatine kinase (CK) concentration [5]. When statins are prescribed as monotherapy, the incidence of myopathy is approximately 0.1-0.5% and is dose-related [6, 7]. The mechanism by which statins cause rhabdmyolysis is not precisely known. Statins are divided into lipophilic and hydrophilic statins. For example, cerivastatin, simvastatin, fluvastatin and atorvastatin are relatively lipophilic, while pravastatin and rosuvastatin are known to be hydrophilic statins. There has been little investigation of pravastatin- and rosuvastatin-induced cytotoxicity. The n-octanol/water partition coefficients of pravastatin at pH 6.8 and 7.4 have been reported to be –0.23 and –0.7, respectively and those of rosuvastatin have been reported to be 0.25 and –0.21, respectively [8]. Metabolic acidosis is most simply defined as a decline in systemic pH induced primarily by a reduction in systemic bicarbonate concentrations. We hypothesized that a condition of acidosis leads to an increase in rosuvastatin and pravastatin intracellular accumulation and induction of muscle damage. Moreover, the effect of medium pH on the cytotoxicity of statins has not been investigated. In the present study, we examined the relationship between pravastatin- and rosuvastatin-induced cytotoxicity and medium pH.
Materials and METHODS
Chemicals
Pravastatin Na and rosuvastatin Ca were kindly donated by Sankyo (Tokyo, Japan). All other reagents were of the highest grade available and used without further purification.
Cell culture
The RD cell line, derived from a human rhabdomyosarcoma and L6 cell line, derived from a rat skeletal muscle were maintained in plastic culture flasks (Corning Incorporated, Corning) as described previously [9, 10]. The RD and L6 cells were kept in Dulbecco's modified Eagle's medium (Sigma) with 10% fetal bovine serum (ICN Biomedicals, Inc., Aurora, OH) and 1% penicillin-streptomycin at 37℃ under 5% CO2.
MTT assay
The 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide (MTT) assay was performed as described previously [11]. The MTT assay relies on the production of a colored formazan by the action of mitochondrial enzymes on MTT. For the MTT assay, RD and L6 cells were seeded at a density of 5 X 103 cells/well on 96-well plastic plates. Following cell attachment (24 h), various concentrations of pravastatin and rosuvastatin were added for the times indicated. At 4 h before the end of treatment, 10 µL of PBS-containing MTT solution (0.5%) was added, and the cells were incubated for a further 4 h. The MTT medium was then replaced with 0.2 ml dimetylsulfoxide, and absorbance was read at 590 nm. Absorbance measured in MTT assays was expressed as percent of the control (defined as 100%).
Uptake study
In the experiments on uptake of pravastatin and rosuvastatin, after removal of the growth medium, cells were washed with HEPES buffer (25 mM D-glucose, 137 mM NaCl, 5.37 mM KCl, 0.3 mM Na2HPO4, 0.44 mM KH2PO4, 4.17 mM NaHCO3, 1.26 mM CaCl2, 0.8 mM MgSO4 and 10 mM HEPES, pH 7.4) and preincubated at 37 °C for 10 min with 0.5 mL of HEPES buffer (pH 7.4). Uptake was initiated by applying HEPES buffer (pH 6.8 or 7.4) containing 100 µM pravastatin and rosuvastatin. The uptake study was performed at 37℃. After a predetermined time period, uptake was terminated by suctioning off the applied solution and immersing the plates in ice-cold HEPES buffer (pH 7.4) and then suspending in 0.5 mL of ice-cold water. Samples were frozen at –80℃ until the assay. The cellular protein content was determined by the method of Lowry et al. with bovine serum albumin as a standard [12].
Rosuvastatin concentrations were determined using an HPLC system as described previously [13]. Pravastatin concentrations were determined using an HPLC system equipped with a UV detector. The column was Inertsil ODS-2 (5 µm, 150 mm × 4.6 mm i.d), and a mobile phase containing 2.5 mM CH3COONH4 : CH3CN (1 : 1, v/v) was used. Column temperature and flow rate were 40 °C and 1.0 ml/min, respectively.
Caspase assay
The caspase assay was performed as described previously [13]. RD and L6 cells were lysed with a cell culture lysis reagent (Promega, Madison, WI). Protein concentration of the cell lysate was adjusted to 5 µg/mL, and the cell lysate was assayed for caspase-3/7 using Ac-DEVD-pNA as colorimetric substrates as described in the manufacturer’s protocol (Promega, Madison, WI).
Data analysis
Student’s t-test was used to determine the significance of differences between two group means. Comparisons between more than two groups were made by using the post-hoc Scheffe test. Statistical significance was defined as P < 0.05.
Results
Effects of medium pH on growth inhibition of pravastatin and rosuvastatin in RD and L6 cells
RD and L6 cells cytotoxicity determined with MTT assay revealed that rosuvastatin moderately reduced the number of viable cells in a concentration-dependent manner, but pravastatin had negligible influence on the viability of the cells (Fig. 1). To clarify the effects of medium pH on pravastatin- and rosuvastatin-induced cytotoxicity, we examined the cytotoxicity of pravastatin and rosuvastatin over the medium pH range of 6.8 to 7.4. As shown in Fig. 2, rosuvastatin cytotoxicity at acidic pH (medium pH 6.8) was significantly greater than that at medium pH 7.4. On the other hand, medium pH had no effect on pravastatin cytotoxicity.
Effects of medium pH on morphology of pravastatin- and rosuvastatin-treated RD and L6 cells
In order to clarify the effects of medium pH on pravastatin- and rosuvastatin-induced cytotoxicity, we examined the morphology of pravastatin- and rosuvastatin-treated RD and L6 cells over the medium pH range of 6.8 and 7.4. As shown in Fig. 3, RD and L6 cells underwent dramatic morphological changes and became smaller after exposure to rosuvastatin at acidic pH (medium pH 6.8) for 48 hours. RD and L6 cells were shrunken and the ratio of nuclear/cytosolic volume increased. In contrast, pravastatin had no effect on cell morphology. These results are identical to those shown in Fig. 2.
Effects of medium pH on accumulation of pravastatin and rosuvastatin in RD and L6 cells
To clarify the difference between the cytotoxicity of pravastatin and that of rosuvastatin, we examined the accumulation of pravastatin and rosuvastatin over the medium pH range of 6.8 to 7.4 in RD and L6 cells. The time courses of accumulation of pravastatin and rosuvastatin reached an equilibrium in 30 min (data not shown). As shown in Fig. 4, medium pH had no effect on pravastatin accumulation. On the other hand, rosuvastatin accumulation at acidic pH (medium pH 6.8) was significantly greater than that at medium pH 7.4. Accordingly, these results suggest that statin-induced cytotoxicity is associated with intracellular accumulation of statins.
Effects of medium pH on caspase activation of pravastatin- and rosuvastatin-treated RD and L6 cells
In order to confirm apoptosis, we examined the activation of caspases of pravastatin- and rosuvastatin-treated RD and L6 cells over the medium pH range of 6.8 to 7.4. The effector caspase 3/7 plays a central role in apoptosis since it translocates from the cytosol into the nucleus upon activation [14]. As shown in Fig. 5, caspase 3/7 activity was significantly increased after exposure to rosuvastatin at acidic pH (medium pH 6.8) for 48 hours. On the other hand, rosuvastatin at medium pH 7.4 did not markedly enhance the activity of caspase 3/7 . Moreover, pravastatin-induced activation of caspase-3/7 at acidic pH (medium pH 6.8) was not significantly greater than that of rosuvastatin-induced activation(data not shown).
Discussion
In terms of the more safety use of statins, the effects of various conditions on statin-induced cytotoxicity must be clarified. However, cytotoxicity of the hydrophilic statins pravastatin and rosuvastatin has not fully investigated. Metabolic acidosis is most simply defined as a decline in systemic pH induced primarily by a reduction in systemic bicarbonate concentrations. It has previously been reported that the lipophilicity of pravastatin and that of rosuvastatin were increased in acidic pH [9].

Figure 1. Effects of pravastatin and rosuvastatin on the viability of RD (a) and L6 (b) cells. Cell viability was measured by the MTT assay. RD and L6 cells were exposed to various concentrations of pravastatin and rosuvastatin for 48 hours. Each point represents the mean with S.D. of 4-6 determinations. *; significantly different from control (no drug) at P < 0.05.
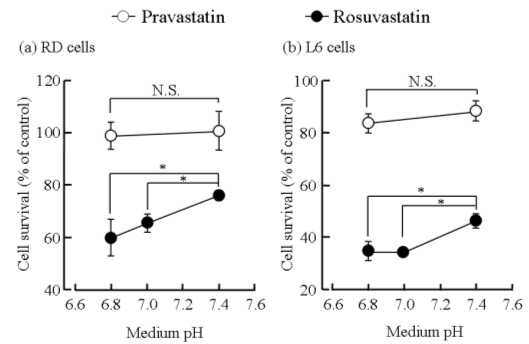
Figure 2. Effects of medium pH on growth inhibition of pravastatin and rosuvastatin in RD (a) and L6 (b) cells. The reduction of RD and L6 cell viability induced by pravastatin (100 µM) and rosuvastatin (100 µM) at each pH for 48 hours was assessed by the MTT assay. Each point represents the mean ± S.D. of 5-6 determinations. *; significantly different from pH 7.4 at P < 0.01
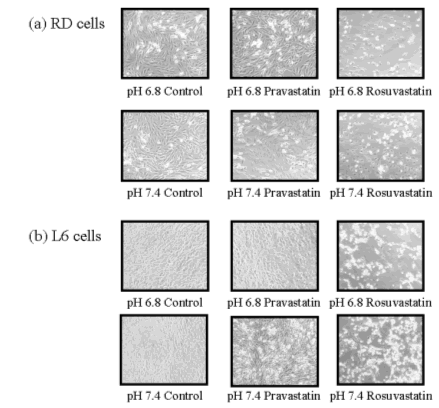
Figure 3. Effects of medium pH on morphological degeneration in pravastatin- and rosuvastatin-treated RD (a) and L6 (b) cells. RD and L6 cells were exposed to pravastatin (100 µM) and rosuvastatin (100 µM) at pH 6.8 and 7.4 for 48 hours, and the cells were examined by microscopy (×100).
Accordingly, it is important to clarify the effect of medium pH on statin-induced cytotoxicity. Firstly, to confirm the effects of medium pH on growth inhibition of pravastatin and rosuvastatin, we examined the effects of statins on viability, morphology and caspase-3/7 activation of RD and L6 cells. Rosuvastatin-induced reduction of cell viability, morphological degeneration and caspase-3/7 activation at acidic pH were significantly greater than those at medium pH 7.4 (Figs.2, 3, 5). On the other hand, medium pH had no effect on those of pravastatin. Rosuvastatin accumulation at acidic pH (medium pH 6.8) was significantly greater than that at medium pH 7.4. On the other hand, medium pH had no effect on pravastatin accumulation (Fig. 4). Accordingly, these results suggest that statin-induced cytotoxicity is associated with intracellular accumulation of statins. We found that rosuvastatin cytotoxicity and pravastatin cytotoxicity were weaker than the cytotoxicity of the lipophilic statin cerivastatin. Moreover, cerivastatin accumulation was greater than that of hydrophilic statins [13]. An acidosis condition, an increased acidity (i.e., increased hydrogen ion concentration) of blood plasma, is said to occur when arterial pH falls below 7.35. Although hydrophilic statins are safer than lipophilic statins, an acidosis condition may lead to a risk of cytotoxicity due to an increase in rosuvastatin accumulation. On the other hand, we speculate that rosuvastatin accumulation in hepatocyte may increase under acidosis condition, leading to potentiate choresterol-lowering effect of tissue rosuvastatin. Further investigations to clarify the effect of pH on hydrophilic statin-induced damage in vivo and examine the rosuvastatin accumulation and its choresterol-lowering effect in hepatocyte in acidic pH are in progress. The results of this study suggest that the rosuvastatin cytotoxicity at acidic pH is associated with increased intracellular accumulation of the drug. On the other hand, medium pH had no effect on cytotoxicity of pravastatin.
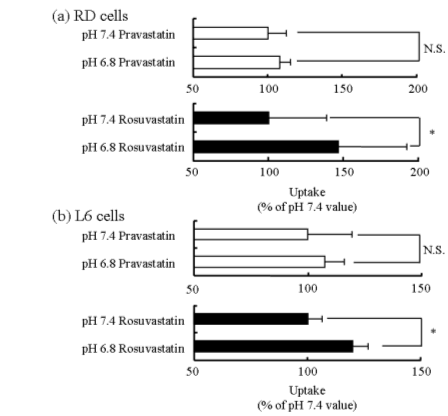
Figure 4. Effects of medium pH on accumulation of pravastatin and rosuvastatin in RD (a) and L6 (b) cells. The accumulation of pravastatin (100 µM) and rosuvastatin (100 µM) in RD and L6 cells was measured at pH 6.8 and 7.4 for 1 hour. Each column represents the mean with S.D. of 3-9 determinations. *; significantly different from pH 7.4 at P < 0.01.

Figure 5. Effects of medium pH on caspase-3/7 activity ratio in rosuvastatin-treated RD (a) and L6 (b) cells. RD and L6 cells were exposed to rosuvastatin (100 µM) at pH 6.8 and 7.4 for 48 hours, and the cell lysate was used to determine the caspase-3/7 activity ratio. Each column represents the mean with S.D. of 3 determinations. *; significantly different from pH 7.4 at P < 0.05
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
| [1] | Havel, R.J., Rapaport, E., Management of primary hyperlipidemia. New Engl J Med,332:1491–1498, 1995. |
| [2] | Jukema, J.W., Bruschke, A.V., van Boven, A.J., Reiber, J.H., Bal, E.T., Zwinderman, A.H., Jansen, H., Boerma, G..J., van Rappard F.M., Lie, K.I. et al., Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation, 91:2528–2540, 1995. |
| [3] | Downs, J.R., Clearfield, M., Weis, S., Whitney, E., Shapiro, D.R., Beere, P.A., Langendorfer, A., Stein, E.A., Kruyer, W., Gotto, A.M.Jr., Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA, 279:1615–1622, 1998. |
| [4] | Omar, M.A., Wilson, J.P., Cox, TS., Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother, 35:1096-1107, 2001. Review. Erratum in: Ann Pharmacother, 35:1296, 2001. |
| [5] | Pedersen, T.R., Kjekshus, J., Berg, K., Haghfelt, T., Faergeman, O., Faergeman, G., Pyorala, K., Miettinen, T., Wilhelmsen, L., Olsson, A.G., Wedel, H., Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler. Suppl, 5:81-87, 2004. |
| [6] | Maron, D.J., Fazio, S., Linton, M.F., Current perspectives on statins. Circulation,101:207-213, 2000. |
| [7] | Garnett, W.R., Interactions with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am J Health Syst Pharm, 52:1639-1645, 1995. |
| [8] | Maeda, N., Yokota, H., Sugiura, M., Kohama, T., Saito, M., Kurihara, A., Tabata, F., Seda, Y., Relationship between water-octanol partition coefficient and inhibition of proliferation of cultured cell lines among various HMG-CoA reductase inhibitors (Statins).Prog Med, 24: 2517-2523, 2004. |
| [9] | Kobayashi, M., Fujita, I., Itagaki, S., Hirano, T., Iseki, K., Transport mechanism for L-lactic acid in human myocytes using human prototypic embryonal rhabdomyosarcoma cell line (RD cells). Biol Pharm Bull, 28:1197-1201, 2005. |
| [10] | Kobayashi, M., Itagaki, S., Hirano, T., Iseki, K., Mechanism of L-lactic acid transport in L6 skeletal muscle cells. Drug Metab Pharmacokinet, 19:363-368, 2004. |
| [11] | Mosmann, T., Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol. Methods, 65:55-63, 1983. |
| [12] | Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., Protein measurement with the folin phenol reagent. J Biol Chem, 193:265-275, 1951. |
| [13] | Kobayashi, M., Kaido, F., Kagawa, T., Itagaki, S., Hirano, T., Iseki, K., Preventive effects of bicarbonate on cerivastatin-induced apoptosis, Int J Pharma in press. |
| [14] | Ferri, K.F., Kroemer, G.., Organelle-specific initiation of cell death pathways, Nat Cell Biol, 3:255-263, 2001. |
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.