J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):340-349, 2007
Protective effects of tungstophosphoric acid and sodium tungstate on chemically induced liver necrosis in wistar rats
Snežana Uskoković-Marković1, Marina Milenković2, Aleksandra Topić3, Jelena Kotur-Stevuljević3, Aleksandra Stefanović3, Jelena Antić-Stanković2
1- Department of Analytical Chemistry, Faculty of Pharmacy, University of Belgrade, Serbia
2- Department of Microbiology and Immunology, Faculty of Pharmacy, University of Belgrade, Serbia
3- Department of Medical Biochemistry, Faculty of Pharmacy, University of Belgrade, Serbia
Received April 20, 2007; Revised July 9, 2007; Accepted July 19, 07; Published July 20, 2007.
Corresponding Author: Snežana Uskoković-Marković, Department of Analytical Chemistry, Faculty of Pharmacy, Vojvode Stepe 450, 11 000 Belgrade, Serbia Tel: +381 11 3970 379, Email: snezaum@pharmacy.bg.ac.yu
ABSTRACT - PURPOSE. Many chemical compounds and infectious agents such as viruses induce liver damage like necrosis or fulminant hepatic failure which is sometimes difficult to manage by medical therapies. The induced liver necrosis by carbon tetrachloride (CCl4) and thioacetamide (TAA) are exemplary models for experimental liver necrosis caused by oxygen free radicals. The aim of this study was to investigate the effects of tungstophosphoric acid (TPA) and sodium tungstate (ST) on liver injury induced by CCl4 or TAA. METHODS. Hepatoprotective effects of TPA and ST on acute liver necrosis, chemically induced, were evaluated by the activity of serum enzymes (alkaline phosphatase, alanine transaminase and aspartate transaminase), oxidative stress parameters (activity of xanthine oxidase, concentrations of malondialdehyde and production of superoxide anion), antioxidative defence markers (concentration of reduced glutathione), and histopathology in Wistar rats. Liver necrosis was induced by administering a single intraperitoneal (i.p.) injection of CCl4 (1.0 ml/kg b.wt. of 80% CCl4 in corn oil) or a single i.p. injection of TAA (400 mg/kg b.w. dissolved in normal saline). TPA and ST were administrated to rats orally for 7 weeks (50 mg/kg b.wt.) prior to induction of liver necrosis. RESULTS. Induced liver necrosis caused significant elevation of activity of liver enzymes, parameters of oxidative stress and marked changes in histopathology, like necrosis of hepatocytes, hepatocyte degeneration and infiltration of inflammatory cells. In TPA and ST pretreated rats histopathological changes were almost absent, serum enzymes and oxidative stress parameters were decreased, while at the same time the concentration of reduced gluthathione was increased. CONCLUSION. The present findings suggest that treatment with TPA and ST for 7 weeks could be useful for the prevention of hepatic injury in rats.
Introduction
Acute hepatic failure and liver necrosis frequently develops upon exposure of the tissue to viruses or numerous chemical agents and represents a complex process, characterized by simultaneous activation of multiple deregulated pathways that culminate in the loss of cell membrane integrity and thus, the leakage of cellular constituents (1). The understanding and treatment of liver necrosis has developed rapidly over the last 50 years. Using animal models that reflect the clinical, biochemical and histological pattern of the syndrome seen in humans progress has been made in reducing morbidity and mortality from this syndrome. Administration of two hepatotoxins, carbon tetrachloride (CCl4) and thioacetamide (TAA) is often used to induce experimental acute liver necrosis in rats. Previous studies have shown that the hepatotoxic effects of CCl4 and TAA involve oxidative stress manifested as a lipid peroxidation, protein oxidation and alterations in tissue antioxidants (2-5).
During the last decades, studies of biomedical activity of inorganic compounds like molybdenum and vanadium compounds have attracted the attention of numerous investigators (6-9). Reviews about bioactivity of tungsten compounds are infrequent (10-12). Tungstate shares a common tetrahedral structure (13) with vanadate, molybdate and phosphate, and this structure seems to be an important characteristic of efficient inhibitors competing with phosphate substrates for binding to the active catalytic site of phosphatases. The literature data confirmed that only oxoanionic molecular species of tungsten W (VI) (such as WO42- in sodium tungstate Na2WO4, ST) are stable under the aqueous conditions (6,7). Tungsten has the ability to form weak acids, readily condensing/polymerizing into anions containing several molecules of an acid anhydride. When these condensed species contain only one type of acid anhydride, they form isopoly compounds. Condensation of the acid with other acids (e.g., phosphoric or silicic), leads to the formation of the ‘polyoxotungstate’, such as Keggin anion, [PW12O40]3-, in 12-tungstophosphoric acid (TPA) . Polyoxotungstates such as TPA are well-known in several application fields, such as analytical reagents, catalysis, nanomaterials, etc., but biomedical activities of TPA are still not well defined.
Much attention has recently been given to ST as a potential compound with biochemical and biological effects based mainly on binding affinity with many substances (14-18). Sodium tungstate therapy is already known and utilized as a non-traditional folk medicine in Japan. Recent studies (19-21) reported antidiabetic and antiobesity properties of ST. Pawa and Ali (22) pointed to the protective role of the ST in liver necrosis and fulminant hepatic failure in rats.
The aim of this study was to investigate protective effects of TPA on induced liver necrosis in adult Wistar rats. In assessing the protective effect of TPA on liver necrosis, we also used ST, which was previously confirmed as a hepatoprotective agent. To our knowledge it is for the first time that hepatoprotective effect of tungstophosphoric acid has been tested.
Materials and METHODS
Animals
Eight weeks old female albino rats of the Wistar strain (weighing 150-180 g) were provided from the Military Medical Academy Animal House, Belgrade. The animals were maintained with commercial rat feed and water available ad libitum. They were housed in polypropylene cages at room temperature 25±2°C (each group contained ten rats) with a wire mesh top and a hygienic bed of soft wood scrapings with proper humane care. Animal studies were conducted in accordance with the institutional guidelines for care and use of laboratory animals.
The animals were divided into nine groups, each of which contained 10 rats. Group 1 was the normal control group (N) in which saline solution and corn oil were given orally. Groups 2 and 3 were rats orally treated with sodium tungstate and tungstophosphoric acid (ST, TPA, respectively). Groups 4 and 5 were the CCl4- and TAA-induced liver necrosis groups (C,T, respectively) in which liver necrosis was induced with a single intraperitoneal injection of hepatotoxin. Groups 6-9 were rats orally treated with sodium tungstate and tungstophosphoric acid prior to induction of liver necrosis by CCl4 and TAA (ST+C, ST+T, TPA+C, TPA+T, respectively).
Chemicals
Chemicals used in this study were of the highest purity. TPA and ST were purchased from Aldrich Chemical Company, Milwaukee, USA. TPA was previously recrystallized twice from bidistilled water. All other chemicals and solvents were of commercial p.a. reagent-grade and were used without further purification.
Induction of liver necrosis
Liver necrosis was induced by administering a single intraperitoneal (i.p.) injection of CCl4 (1.0 ml/kg b.wt. of 80% CCl4 in corn oil) or a single i.p. injection of TAA (400 mg/kg b.w. dissolved in normal saline).
To evaluate and compare the protective roles of TPA and ST against hepatic lesions produced by TAA and CCl4, they were administrated to rats orally for 7 weeks (50 mg/kg b.wt.) followed by necrogenic dose of each hepatotoxin.
Processing of the liver tissue and biochemical estimations
Liver injury was evaluated by analyzing serum/tissue lysate obtained from rats sacrificed 24h after receiving the necrogenic dose of hepatotoxins. Rats were anesthetized with ether and the blood was drawn by puncture from vena cava inferior. Blood was allowed to clot and the serum was separated by centrifugation at 1200 x g for 10 min. Serum was used to analyze activity of liver enzymes alkaline phosphatase and transaminases. Other biochemical estimations were performed on a tissue homogenate prepared from the liver.
Each animal was weighed before sacrificing. Total liver weight of rats from each group was also recorded before the tissue was processed for analyses. The livers of the rats were removed, washed in ice-cold saline solution, blotted and a small portion was cut and weighed for homogenization. Tissue lisate was prepared in nine volumes of 0.1 M phosphate buffer (pH 7.4) containing 1.15% KCl, using a homogenizer. A portion of the homogenate was kept for determination of hepatic malondialdehyde, superoxide anion and reduced glutathione content. The rest of the homogenate was subjected to centrifugation in a cooling centrifuge initially at 800 x g for 10 min (to remove the nuclei and other cell debris) and then the resultant supernatant was centrifuged at 9500 x g for 20 min to get the post-mitochondrial supernatant (used to measure activity of xanthine oxidase).
Activities of alanine transaminase (ALT) and aspartate trasaminase (AST) and alkaline phosphatase (AP) were measured using diagnostic kits (Randox Laboratories, UK), and enzyme activity was expressed in U/L.
To determine the index of oxidative stress in the liver tissue, we used the thiobarbituric acid reacting substances (TBARs) test that measures the quantity of the malondialdehyde (MDA) product as by Girotti et al (23). MDA concentration is expressed as nmol/g of liver protein. Production of superoxide anion (O2.-) as a marker of the first stage of oxidative stress was measured as described by Auclair and Voisin (24) at pH 7 and 25°C from the rate of nitroblue tetrazolium (NBT) reduction. Results are expressed as nmol NBT/min/g of liver protein. Reduced glutathione (GSH) content was measured according to method of Jollow et al. (25). Results are expressed as nmol GSH/g of liver protein. Xanthine oxidase (XO) in postmitochondrial supernatant (PMS) was assayed according to Stirpe-Della Corte method (26). Results are expressed as mmol uric acid/g of PMS protein.
The total protein concentration in all tissue samples was assayed according to Bradford’s method (27).
Histopathology
Hepatic morphology was assessed by light microscopy. The left lateral lobe of the liver was sliced (three slices per rat) fixed in 10 % buffered-neutral formalin and embedded in paraffin. Transverse sections of 5 mm in thickness were subjected to hematoxylin and eosin (H&E) staining before examinations (28). The sections were scanned and analyzed by a certified pathologist who was not aware of sample assignment to experimental groups. A minimum of 10 fields was scored per liver slice to obtain the mean value. To quantify the morphological changes, liver sections were graded for necrosis using an arbitrary scale: 0-negative findings (0 %); 1-slight (about 20-30 % necrosis); 2-moderate (about 50 % necrosis); 3-marked (about 75 % necrosis); and 4-very intense (about 90-100 % necrosis) (29).
Statistical analysis
All data were expressed as mean ± standard error of mean (S.E.M). Differences between the hepatotoxin treated group and tungstate-pretreated groups were assessed by the Mann-Whitney U-test. P < 0.05 was considered to be significant..
Results
Effects of tungstophosphoric acid and sodium tungstate on liver histology and liver weight/body weight ratio
The effects of TPA and ST on the acute liver necrosis induced by CCl4 or TAA were assessed by histopathological examinations. The hematoxylin and eosin staining of liver specimens is demonstrated in Figure 1.
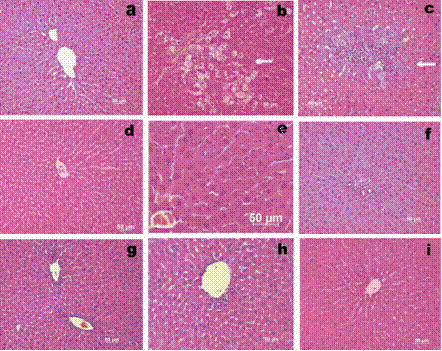
Figure 1. The representative hepatic histopathology: a) Normal liver from normal (healthy) untreated control rat. b) Severe centrilobular necrosis and infiltration in rats treated with CCl4 . c) Necrosis and mononuclear cell infiltration in rats treated with TAA. d) Rats pretreated with TPA alone. Normal appearance of liver. e) Rats pretreated with TPA followed by CCl4. Normal appearance of liver. f) Rats pretreated with TPA followed by TAA. Normal appearance of liver. g) Rats treated with ST alone. Normal appearance of liver. h) Rats pretreated with ST followed by CCl4. Normal appearance of liver. i) Rats pretreated with ST followed by TAA. Normal appearance of liver. Hematoxylin and eosin (H&E) staining (200x).
There are no pathological changes in healthy control livers which showed normal lobular architecture with central vein and radiating hepatic cords (Figure 1a). Rats treated with tungstate alone showed no sign of damage (Figure 1 d,g) when compared to the normal control. Hepatic cell death induced by the specified doses of CCl4 or TAA is observed to be restricted to necrosis. Apoptotic bodies were not detected in any of the slides. In CCl4 treated rats, sections show necrosis of hepatocytes, hepatocyte degeneration and infiltration of inflammatory cells. There was marked neutrophilic and mononuclear cell infiltration with severe centrilobular congestion (Figure 1 b). Bridging necrosis was present in certain cases. Changes improved in TPA pretreated rats, which exhibited areas of normal liver architecture and patches of necrotic hepatocytes (Figure 1 e). The liver section of TAA treated rats also shows necrosis of hepatocytes with liver architecture destruction, and inflammatory infiltrate in the portal tracts (Figure 1 c). These changes were almost absent (except for a few necrotic areas) in sections of liver obtained from rats receiving TPA and ST followed by TAA (Figure 1 f,i). The liver weight/body weight ratio is presented in Table 1 in experimental groups. Only in ST treated rats was the liver weight/body weight ratio lower than for the normal control.
Effects of tungstophosphoric acid and sodium tungstate on biochemical parameters associated with liver necrosis
The serum activities of ALT, AST and AP were used as biochemical markers for the early acute hepatic damage. In TPA and ST treated rats the activity of these parameters was not different comparing with normal untreated rats (N). Necrosis score (NS) and activity of liver enzymes in CCl4 and TAA treated rats were significantly higher than in normal control (Figure 2 a,b,c,d). In rats pretreatred with tungsten compounds followed by hepatotoxins (TPA+C, ST+C, TPA+T) liver enzymes and necrosis score significantly decreased. In ST+T group activity of liver enzymes and necrosis score showed a trend to decreasing (not statistically significant) when compared with T group.
Table 1. Liver weight/body weight ratios in rats
Experimental |
Liver weight / |
N |
3.73 ± 0.32 |
C |
3.62 ± 0.17 |
T |
3.38 ± 0.15 |
TPA |
3.33 ± 0.15 |
ST |
2.88 ± 0.06** |
TPA+C |
3.35 ± 0.26 |
TPA+T |
3.29 ± 0.08 |
ST+C |
3.82 ± 0.14 |
ST+T |
3.63 ± 0.22 |
* N: normal control, rats without any treatment; C,T: CCl4- and TAA-induced liver necrosis rats; TPA, ST: rats orally treated with tungstophosphoric acid and sodium tungstate; TPA+C, TPA+T, ST+C, ST+T: rats orally treated with tungstophosphoric acid and sodium tungstate prior to induction of liver necrosis by CCl4 and TAA respectively.
** significantly different from normal control, P<0.05.
In TPA- and ST-treated rats, the activity of XO was not different comparing to the normal-untreated rats (Figure 3 a). Levels of XO in CCl4 treated rats were significantly higher than in the normal control. In TAA treated rats, XO was slightly elevated. Activity of XO in TPA and ST pretreated rats followed by CCl4 induced necrosis, and TPA pretreated rats followed by TAA induced necrosis, were significantly lower than in rats with liver necrosis.
Both the hepatotoxin treated groups (Figure 3 b,c) showed elevation of oxidative stress parameters, O2.¯and MDA comparing to normal, untreated rats. Significant decrease of O2·¯ was detected in TPA and ST pretreated groups followed by TAA induced liver necrosis. But, in tungsten compounds pretreated groups followed by CCl4 induced liver necrosis there was a tendency to decreasing production of O2·¯. The concentration of MDA in all pretreated groups followed by both hepatotoxins showed tendency to decreasing, but not statistically significant.
Significant depletion of reduced glutathione (GSH) was detected in CCl4 and TAA treated rats (Figure 3 c). Pretreatment with TPA or ST leads to significant rise in hepatic GSH level. Increasing of the GSH in CCl4 induced necrosis was more efficient by ST pretreatment than by TPA pretreatment. However, in TAA induced liver necrosis, TPA pretreatment was more efficient in elevation of GSH.
Discussion
A number of drugs, chemicals and viruses have been reported to cause severe liver necrosis, which sometimes becomes difficult to manage by medical therapies. It is important to search for compounds that can be used for better management of the hepatic failure due to severe necrosis.
The present study was designed to evaluate whether pretreatment with different compounds of tungsten (TPA and ST) would have a hepatoprotective effect on chemically induced liver necrosis. It has been already reported that ST could protect progression of CCl4 and TAA induced hepatic injury (22). However, the in vivo hepatoprotective activity of TPA remains unknown.
Rats orally treated with TPA as well as ST (50 mg/kg b.wt.) during the 7 weeks did not have changes of liver enzymes and hepatic histology (Figure 1 d,g) when compared to the normal control (Figure 1 a). But we detected decreasing of liver weight/body weight ratio in ST treated rats compared to untreated rats (Table 1).
In this study, to induce liver damage, we used compounds which mediate their hepatotoxicity via the formation of free radicals, especially the reactive oxygen species (ROS). CCl4 and TAA induced toxicity as experimental models are well-characterized (2, 4). The hepatotoxicity of both compounds results from their metabolism by the cytochrome P450 enzyme system into the reactive intermediates, which leads to oxidative stress and causes hepatic necrosis. The necrogenic doses of CCl4 and TAA which have been given to rats significantly elevated necrosis score and liver enzymes (Figure 2 a,b,c,d). These results were consistent with the literature (2,5). Also, this study has shown that prophylactic treatment of rats with TPA and with ST, before the induction of liver injury, effectively alleviates the liver injury as indicated by significant decrease necrosis score and liver enzymes (Figure 2 a,b,c,d).
..... 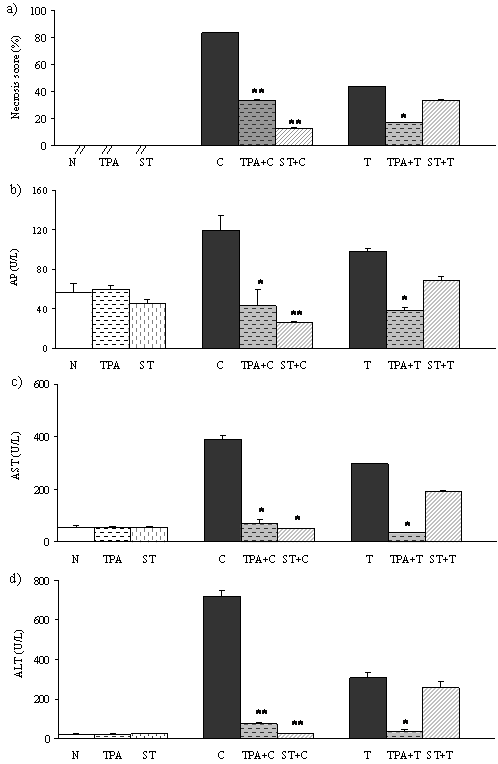
Figure 2. Effects of TPA and ST on biochemical parameters associated with liver necrosis. Data are expressed as means ± S.E.M. (n=10). ST and TPA-pretreated groups were compared with hepatotoxin treated group. The level of statistical significance was:*P<0.05 and ** P<0.001. a) necrosis score (%); b) activity of alkaline phosphatase (U/L); c) activity of aspartate transaminase (U/L); d) activity of alanine transaminase (U/L).
Effects of tungstophosphoric acid and sodium tungstate on biochemical parameters associated with liver necrosis as a result of oxidative stress
The results of oxidative stress parameters and antioxidative defence in the liver in rats are shown in Figure 3 a,b,c,d.
.....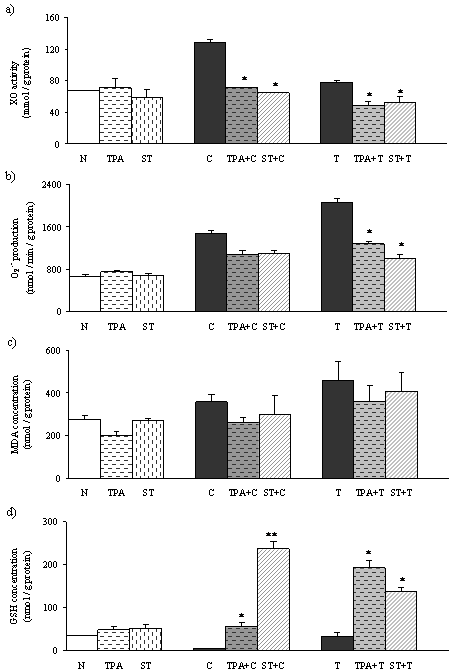
Figure 3. Effects of TPA and ST on oxidative stress parameters and antioxidative defence status Data are expressed as means ± S.E.M (n=10). ST and TPA-pretreated groups were compared with hepatotoxin-treated group. The level of statistical significance was: *P<0.05 and ** P<0.001. a) activity of xanthine oxidase (mmol/g protein); b) production of superoxide anion (nmol/min/g protein); c) malondialdehyde (nmol/g protein.); d) reduced glutathione (nmol/g protein).
Histopathological data also pointed towards beneficial effects of TPA and ST in ameliorating liver damage induced by CCl4 and TAA (Figure1). In CCl4 induced hepatic injury ST was more efficient in hepatoprotection than TPA, while in TAA induced hepatic injury, TPA was more efficient than ST.
The involvement of xanthine oxidase in ROS-mediated diseases has been proposed as a result of the generation of O2.¯ and H2O2 during hypoxanthine and xanthine oxidation. Xanthine oxydoreductase is molybdenum-containing monooxygenase and exists as dehydrogenase (XDH) and oxidase (XO). The XDH prefers NAD+ as an electron acceptor, but will use molecular oxygen in the absence of NAD+. The XO utilizes oxygen as an electron acceptor and has very little activity toward NAD+ which promotes generation of ROS. Under physiological conditions, the enzyme is active as XDH, but several stimuli such as inflammation and hypoxia promote the conversion of XDH to the XO form. Moreover, it was shown (30) that, in pathological conditions, H2O2 derived from the NADPH oxidase markedly enhances the irreversible conversion of XDH to XO, leading to ROS production.
To evaluate the involvement of oxygen radicals in hepatic damage and potential defence of tungsten compounds, we have measured activity of XO. In this study we have found that CCl4 and TAA induced necrosis provokes XO activity which produces oxidative stress by generating ROS (Figure 3a), indicating its role in this type of liver injury. Significant decrease of XO activity was observed in ST pretreated rats followed by hepatotoxins (Figure 3a), which is in accordance with results of Pawa and Ali (22). Recent studies reported that ST prevents molybdenum incorporation into the active centre of XDH/XO as a competitive inhibitor and provokes decrease of XO activity (31-32). Further, we detected decrease of XO activity in TPA treated rats, which could explain the hepatoprotective effect of TPA by the same mechanism as in ST pretreatment.
Superoxide anion radical O2.¯ is produced in aerobic metabolism, and imbalance between the production and detoxification results in oxidative stress. O2.¯ reacts with polyunsaturated fatty acids to induce the release of toxic and reactive aldehyde metabolites such as malondialdehyde, one of the end products of lipid peroxidation. As a result of increased activity of XO and the activity of the other ROS-generating sources, we obtained increased production of O2.¯ in hepatotoxin treated groups compared with normal control. Suppression of XO activity by both tungsten compounds followed by TAA induced necrosis resulted in decrease of O2.¯ production (Figure 3 b). Pretreatment with TPA or with ST in CCl4 induced necrosis showed tendency to reduce production of O2.¯ but not significantly (Figure 3 b).
Increase in hepatic MDA concentration was detected, as an index of oxidative liver damage, in CCl4 and TAA treated rats (Figure 3 c), which supports the role of XO-derived ROS in their hepatotoxicity. We observed reduction of MDA concentration by both tungsten compounds, but not statistically significant.
The liver injury is often mediated by free radicals or by the depletion of endogenous pool of antioxidants such as GSH. Many authors reported that reduced glutathione (GSH) plays a vital role in cellular function. GSH effectively scavenges free radicals and other reactive oxygen species (e.g. hydroxyl radical, lipid peroxyl radical, peroxynitrite and H2O2) directly and indirectly through enzymatic reactions (33). In such reactions, GSH is oxidized to form GSSG, which is then reduced to GSH by the NADPH-dependant glutathione reductase. In addition, glutathione peroxidase (a selenium-containing enzyme) catalyzes the GSH-dependant reduction of H2O2 and other peroxides and protects the organism from oxidative damage. Glutathione detoxifies toxic metabolites of drugs, regulates gene expression, apoptosis and transmembrane transport of organic solutes. It is essential to maintain the reduced status of the cell/tissue (34), and its severe depletion is reported to lead to liver injury (35).
We obtained significant depletion of GSH level in the liver after injection of both hepatotoxins comparing to normal control rats. This result supports the view that oxidative stress takes place extensively in the liver during necrosis. The oral administration of the ST and TPA prior to the induction of liver necrosis resulted in increase of the hepatocyte GSH (Figure 3 c.). Inhibition of XO and reduction of ROS in ST and TPA pretreatment led to elevation of GSH because the pool of this antioxidant in the liver remained unused. This could provide an explanation for the manifold increasing in hepatic GSH content in the rats which were pretreated with tungstate compounds followed by hepatotoxins. Interestingly, ST compound was more potent in increasing GSH concentration in CCl4 induced hepatic injury, which could explain better the protective effect of this tungstate compound in CCl4 induced hepatic injury (Figure 3 d). In TAA induced hepatic injury, TPA was more efficient in increasing GSH which could explain improvement in histological and biochemical parameters of liver necrosis.
In conclusion, the present findings suggest that tungstophosphoric acid, as well as sodium tungstate, have protective effects on chemically induced liver necrosis which could be explained by their antioxidant properties. Therefore, further work is needed to investigate usefulness of these inorganic compounds in the prevention of chemically induced hepatic injury.
References
| [1] | Lee WR. Acute liver failure. N Engl J Med 1993; 329: 1862-1872. |
| [2] | Williams AT, Burk RF. Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Seminars in Liver Disease 1990; 10: 279-284. |
| [3] | Recknagel RO, Glende EE, Dolak JA, Waller RL. Mechanism of carbon tetrachloride toxicity. Pharmacol Ther 1989; 43: 134-154. |
| [4] | Porter WR, Neal RA. Metabolism of thioacetamide and thioacetamide S-oxide by rat liver microsomes. Drug Metab Dispos 1978; 6: 379-388. |
| [5] | Ali S, Diwakar G, Pawa S, Siddiqui, MR, Jain SK, Abdulla M. Attenuation by boron supplementation of the biochemical changes associated with thioacetamide-induced hepatic lesions. Trace Elem Exp Med 2002; 15: 47- 55. |
| [6] | Delgado O, Dress A, Müller A, Pope MT. Polyoxometalates: A class of compounds with remarkable topology. In: Pope MT, Müller A, ed. Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity, Kluwer Academic Publishers, Dordrecht, 1994. |
| [7] | Rhule J, Hill C, Judd D, Schinazi R. Polyoxometalates in medicine. Chem Rev 1998; 98: 327-357. |
| [8] | Rehder D. The coordination chemistry of vanadium as related to its biological functions. Coord Chem Rev 1999; 182: 297–322. |
| [9] | Stankiewicz PJ, Tracey AS, Crans DC. Vanadium and its role in life. In: Sigel H, Sigel A, ed. Metal Ions in Biological Systems, vol.31, Marcel Dekker, New York, 1995. |
| [10] | Tajima Y. A review of the biological and biochemical effects of tungsten compounds. Curr Top Biochem Res 2001; 4: 129-136. |
| [11] | Yamase T, Fukuda N, Tajima Y. Synergistic effect of polyoxotungstates in combination with β-lactam antibiotics on antibacterial activity against methicillin-resistant Staphylococcus aureus. Biol Pharmacol Bull 1996; 19: 459–465. |
| [12] | Jelikić-Stankov M, Uskoković-Marković S, Holclajtner-Antunović I, Todorović M, Djurdjević P. Compounds of Mo, V and W in biochemistry and their biomedical activity. J Trace Elem Med Biol 2007; 21: 8-16. |
| [13] | Greenwood NN, Earnshaw A. Chemistry of the Elements. Pergamon, New York, 1984. |
| [14] | Matsumoto J.Vanadate, molybdate and tungstate for orthomolecular medicine. Med Hypotheses 1994; 43: 177-82. |
| [15] | Fessenden-Raden JM. Effect of silicotungstate on reduced diphosphopyridine nucleotide oxidation in submitochondrial Particles. J Biol Chem 1971; 246: 6745-6749. |
| [16] | Pitt RM, McKelvey TG, Saenger JS, Shah AK, Jones HP, Manci EA, Powell RW.A tungsten-supplemented diet delivered by transplacental and breast-feeding routes lowers intestinal xanthine oxidase activity and affords cytoprotection in ischemia-reperfusion injury to the small intestine. J Pediatr Surg 1991; 26: 930-935. |
| [17] | Hwang PL, Ryan RJ. Tungstate stimulates adenylate cyclase. Endocrinology 1981; 108: 435-439. |
| [18] | May HD, Patel PS, Ferry JG.Effect of molybdenum and tungsten on synthesis and composition of formate dehydrogenase in methanobacterium formicicum. J Bacteriol 1988; 170: 3384 - 3389.Foster JD, Young SE, Brandt TD, Nordlie RC. Tungstate: A potent inhibitor of multifunctional glucose-6-phosphatase. Arch Biochem Biophys 1998; 354: 125–132. |
| [19] | Foster JD, Young SE, Brandt TD, Nordlie RC. Tungstate: A potent inhibitor of multifunctional glucose-6-phosphatase. Arch Biochem Biophys 1998; 354: 125–132. |
| [20] | Barbera A, Gomis RR, Prats N, Rodriguez-Gil JE, Domingo M, Gomis R, Guinovart JJ. Tungstate is an effective antidiabetic agent in streptozotocin-induced diabetic rats: a long-term study. Diabetologia 2001; 44: 507-513. |
| [21] | Claret M, Corominola H, Canals I, Saura J, Barcelo-Batllori S, Guinovart JJ, Gomis R. Tungstate decreases weight gain and adiposity in obese rats through increased thermogenesis and lipid oxidation. Endocrinology 2005; 146: 4362–4369. |
| [22] | Pawa S, Ali S. Liver necrosis and fulminant hepatic failure in rats: protection by oxyanionic form of tungsten. Biochim Biophys Acta 2004; 1688: 210–222. |
| [23] | Girotti MJ, Khan N, McLellan BA. Early measurement of systemic lipid peroxidation products in plasma of major blunt trauma patients. J Trauma 1991; 31: 32-35.Auclair C, Voisin E. Nitroblue tetrazolium reduction. In: Greenwald RE, ed. CRC Handbook of Methods for Oxygen Radical research. Boca Raton, CRC Press, FL, 1985. |
| [24] | Auclair C, Voisin E. Nitroblue tetrazolium reduction. In: Greenwald RE, ed. CRC Handbook of Methods for Oxygen Radical research. Boca Raton, CRC Press, FL, 1985. |
| [25] | Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974; 11: 151-169. |
| [26] | Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxydase (type O). J Biol Chem 1969; 244: 3855-3863. |
| [27] | Bradford MM. A rapid and sensitive method for the quantitation of the microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976; 72: 248-254. |
| [28] | Kang KW, Choi SH, Ha JR, Kim CW, Kim SG. Inhibition dimethylnitrosamine-induced liver fibrosis by [5-(2-pyrazynil)-4-methyl-1,2-dithiol-3-thione] (oltipraz) in rats: suppression of transforming growth factor- beta 1 and tumor necrosis factor-alpha expression. Chem Biol Interac 2002; 139: 61-77. |
| [29] | De Ferreiyra EC, Bernacchi AS, San Martin MF, Castro GD, Castro JA. Trifluopromazine late preventive effects on Carbon Tetrachloride-Induced Liver Necrosis. Exp Mol Pathol 1995; 62: 75-82. |
| [30] | McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 2003; 285: 2290-2297. |
| [31] | Johnson JL, Rajagopalan KV, Cohen HJ. Effect of tungsten on xanthine oxidase and sulfitr oxidase in the rat. J Biol Chem 1974; 249: 859-866. |
| [32] | Higgins ES, Richert DA, Westerfeld WW. Molybdenum deficiency and tungstate inhibition studies. J Nutr 1956; 59: 539-559. |
| [33] | Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition 2002; 18: 872-879. |
| [34] | Lauterburg BH. Analgesics and glutathione. Am J Ther 2002; 9: 225-233. |
| [35] | Comporti M, Macllaro E, Del Bello B, Casini AF. Glutathione depletion: its effects on other antioxidant systems and hepatocellular damage. Xenobiotica 1991; 21: 1067-1076. |
Published by the Canadian Society for Pharmaceutical Sciences.
Copyright © 1998 by the Canadian Society for Pharmaceutical Sciences.