J Pharm Pharmaceut Sci (www.cspscanada.org) 10(3):299-310, 2007
Cephalexin inhibits N-formylated peptide transport and intestinal hyperpermeability in Caco2 cells
David R. Foster*, and Xiaomei Zheng
Department of Pharmacy Practice, Purdue University School of Pharmacy and Pharmaceutical Sciences, Indianapolis, IN
Date Received October 12,2006; Revised April 2, 2007; Accepted June 12 2007; Published June 13, 2007
Corresponding Author: David R. Foster, Department of Pharmacy Practice, Purdue University School of Pharmacy and Pharmaceutical Sciences, W7555 Myers Building, WHS, 1001 W. 10th Street, Indianapolis, IN, United States, 317-613-2315, Email: drfoster@iupui.edu
ABSTRACT - Purpose. Intestinal barrier integrity is diminished in critical illness and inflammatory bowel disease. Bacterial-derived N-formylated peptides, absorbed by the intestinal oligopeptide transporter, hPEPT1, are involved in the pathogenesis of disease-induced intestinal barrier dysfunction, via stimulation of polymorphonuclear leukocyte (PMN) migration. The purpose of this study was to determine if the hPEPT1 substrate, cephalexin, inhibits the absorption of the N-formylated peptide, N-formyl-L-methionyl-L-leucyl-L-phenylalanine (“fMLP”), thereby preventing hyperpermeability in Caco2 cells. Methods. Caco2 monolayers were grown on permeable supports. fMLP (0.1 µM) was added to apical chambers with and without cephalexin (5 and 10 mM), and fMLP effective permeability was calculated. To determine the ability of cephalexin to attenuate intestinal dysfunction, Caco2 cells were co-cultured with human PMN’s in the presence of fMLP, cephalexin, and inflammatory cytokines. Monolayer integrity was assessed by measuring mannitol permeability. Results. Cephalexin 10 mM significantly reduced fMLP permeability (p=0.007). Monolayer integrity (as indicated mannitol permeability) was decreased in cultures treated with inflammatory cytokines and fMLP, an effect that was attenuated by cephalexin (p<0.01). Conclusion. Cephalexin inhibits fMLP transport across cultured intestinal monolayers, and partially attenuates PMN-induced intestinal hyperpermeability. The use of pharmacologic hPEPT1 substrates may represent a novel means of preserving intestinal barrier integrity.
Introduction
The integrity of the intestinal barrier is diminished as a result of a number of pathological conditions, including sepsis, trauma, burn injury, inflammatory bowel disease, and others (1-3). Importantly, this intestinal hyperpermeability is associated with extensive sequelae, and plays a pivotal role in maintaining states of chronic inflammation and fostering the development of systemic infectious complications. For example, increased paracellular and transcellular permeation of inflammatory stimuli (e.g., endotoxin) may be a contributing factor to the development of the systemic inflammatory response seen in critical illness. Moreover, intestinal translocation of enteric bacteria is thought to be a significant cause of infectious complications and inflammation in critical illness (4). In conditions such as inflammatory bowel disease, there is evidence that the immune system reacts abnormally to the normal intestinal flora (5,6). Although the precise etiology of increased gut permeability remains unknown, a number of studies have demonstrated that inflammatory cytokines, including interferon-γ (IFN-γ), interleukins 1β, and 6 (IL-1β, IL-6) and tumor necrosis factor (TNF) play an important role in mediating intestinal dysfunction (7-9). A complex interrelationship exists between inflammatory stimuli, cytokines, and other integral components of the inflammatory cascade, including the inducible form of nitric oxide synthase (iNOS, the enzyme responsible for nitric oxide [NO] production), all of which may act to affect mucosal integrity (9,10). NO is a pluripotent signaling and effector molecule, and iNOS-mediated NO production has been implicated as a key event in cytokine induced intestinal hyperpermeability. This may occur, in part, via the formation of peroxynitrate, which subsequently causes ATP depletion, cytoskeletal derangements, and hyperpermeability (11,12). Proinflammatory N-formylated peptides produced by anaerobic and aerobic intestinal bacteria are particularly important in mucosal immune activation (13). N-formyl-L-methionyl-L-leucyl-L-phenylalanine (“fMLP”), the most prominent of these peptides, stimulates many leukocyte functions, most notably, chemotaxis (13-15). In addition to triggering receptor mediated chemotaxis of neutrophils, fMLP also activates the 5-lipoxygenase pathway and causes lysosomal enzyme release in neutrophils and monocytes (16,17). Moreover, interactions between fMLP and leucocytes may directly impair intestinal barrier function (14,18). For example, the activation of monocytes by fMLP has been shown to potentiate the intestinal epithelial dysfunction evoked by monocytes treated with TNF (19). fMLP also causes polymorphonuclear leukocyte (PMN) migration across the intestinal cell wall and induces production of neutrophil-derived oxidants, both of which are associated with increases in intestinal permeability (14,18,20). Colonic instillation of fMLP has been used to induce an animal model of mucosal intestinal inflammation, and fMLP is one of the bacterial derived factors implicated in the initiation and perturbation of inflammatory bowel disease (21-23).
The binding sites for fMLP are submucosal; that is, unlike other proinflammatory molecules, intestinal epithelial cells do not express binding sites for fMLP, and fMLP must be absorbed before it can act on neutrophils and monocytes in the lamina propria (24). In the gut, fMLP is transported by hPEPT1, an important intestinal oligopeptide transporter (25). In addition to the absorption of dietary peptides, hPEPT1 is also responsible for the active absorption of many drug substrates, including b-lactam antibiotics (26). No studies have evaluated the potential ability of pharmacologic hPEPT1 substrates to prevent fMLP related intestinal hyperpermeability. The purpose of this study was to determine if a pharmacologic hPEPT1 substrate, cephalexin, inhibits the absorption of the N-formylated peptide, fMLP, thereby preventing intestinal dysfunction in cultured human intestinal monolayers (Caco2 cells).
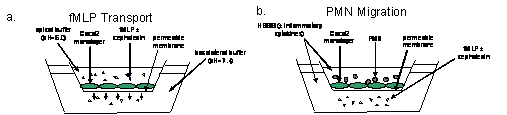
Figure 1. Overview of experimental design. In fMLP transport experiments (figure 1a.), 3H fMLP was added to the apical chambers of permeable supports, with and without cephalexin (also added to the apical chambers). Basolateral concentrations of 3H fMLP were sampled at 0, 15, 30, 45, 60, and 120 minutes. In polymorphonuclear leukocyte (PMN) migration experiments (figure 1b.), polymorphonuclear leukocytes were added to apical chambers of permeable supports in the presence and absence of inflammatory cytokines (IFN-γ, IL-1β, TNF). Control media or media containing fMLP with and without cephalexin was added to basolateral chambers. Cultures were incubated for two hours, and permeability was assessed as described in “Methods” section.
Materials and METHODS
Materials
Caco2 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Dulbecco’s modified eagle’s medium (containing 4.5 g/L glucose and L-glutamine), non-essential amino acids, HEPES, penicillin-G/streptomycin, sodium pyruvate, fetal bovine serum (FBS), phosphate buffered saline (PBS), Trypsin-EDTA, cephalexin, fMLP, 3H fMLP, and 3H mannitol were obtained from Sigma Chemical (St. Louis, MO). Hanks’ balanced salt solution (HBSS) was obtained from BioWhittaker (Walkersville, MD). Sterile collagen-coated permeable cell culture inserts (Transwell®, 3 m pore size) were manufactured by Corning Costar (Corning, NY). ScintiSafe Econo 1 Cocktail was from Fisher Scientific (Fairlawn, NJ). Recombinant human TNF, IL-1b, and IFN-γ were purchased from Peprotech (Rocky Hill, NJ). PMN isolation media (1-Step® Polymorphs) was obtained from Accurate Chemical and Scientific (Westbury, NY). Enzyme-linked immune assay (“ELISA”) kits for quantification of TNF, IL-1β, and IL-6 were obtained from BD Biosciences (San Jose, CA). 10% tris-glycine gels were obtained from Invitrogen (Carisbad, CA). Rabbit polyclonal anti-NOS2 (iNOS) lgG and goat anti-rabbit HRP linked lgG were obtained from Santa Cruz Biotechnology (Santa Cruz, California). Kodak BioMax Light Film (Kodak Co., Rochester, NY) was used for Western blots. All other chemicals and reagents were of the highest grade.
Caco2 Culture Conditions
Caco2 cells (passage 20-30) were cultured at 37oC in 5% CO2 in 100 mm culture dishes, with Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin and 0.1 mg/L streptomycin. Growth medium was replaced thrice-weekly. After achieving confluence in 100 mm culture dishes (5-7 days), cell monolayers were disrupted using trypsin-versine and seeded onto permeable collagen-coated polyester supports at a density of 100,000 cells/ml. Cells were grown for 14-21 days prior to experiments, in order to ensure full differentiation. On the day of experiments, transepithelial electrical resistance (TEER) was measured in each well using a epithelial ohmmeter (World Precision Instruments Inc., Sarasota, FL); wells with TEER ³ 200 Ω ∙cm2 were used in experiments.
fMLP Transport
To determine the effects of cephalexin on the hPEPT1 mediated permeability of fMLP, experiments were conducted to determine fMLP transport in the presence and absence of cephalexin (figure 1a.). Specifically radiolabeled fMLP (3H fMLP, 0.1 mM) was added to the apical chambers of permeable supports, with and without cephalexin 10 mM (also added to the apical chambers). Basolateral concentrations of 3H fMLP were sampled at 0, 15, 30, 45, 60, and 120 minutes, and fMLP concentrations were determined via scintigraphy (Tri-Carb 2100 TR liquid scintillation analyzer, Packard Instrument Co., Meriden, CT). Effective permeability (Peff) was used to describe fMLP transport across the monolayer. Briefly, permeability describes the flux of a molecule across the intestinal wall based on the rate of absorption.(27) Peff of fMLP was calculated using equation 1:
Equation 1: Peff = dC/dt × V/CoAr
where V is the volume of the receiver chamber, C0 is the initial drug concentration in donor chamber, Ar is the surface area of the membrane that separates chambers A and B, and dC/dt is the permeability rate (the slope of plot of concentration in the receiver chamber versus time).
Polymorphonuclear Leukocyte (PMN) Co-Culture
PMN’s were isolated from whole blood colleted form healthy human volunteers using a modified dextran separation technique per the manufacturer’s instructions (1-Step® Polymorphs). PMN’s were washed in HBSS, counted, and subsequently resuspended in HBSS. PMN’s were used within two hours of isolation. This protocol was approved by the Indiana University Purdue University Indianapolis Institutional Review Board (IRB).
fMLP-Induced Intestinal Hyperpermeability
PMN’s (7 x 106 cells/ml) were added to apical chambers of 24 mm permeable supports in the presence and absence of a mix of inflammatory cytokines (IFN-γ 1000 units/ml, IL-1β 1 ng/ml, TNF 10 ng/ml). Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers, and cultures were incubated for two hours (figure 1b.). TEER was measured prior to and following treatment. Subsequently, radiolabeled mannitol (3H mannitol, 50 nM) was added to apical chambers, basolateral concentrations of mannitol were measured after two hours via scintigraphy, and mannitol Peff was calculated as described above (equation 1.).
iNOS Expression and Cytokine Production
To characterize the effects of cephalexin on fMLP-induced intestinal inflammation, iNOS expression and production of the cytokines TNF, IL-1β, and IL-6 by Caco2 cells co-cultured with PMN’s was determined. Expression of iNOS was determined via Western blotting (9). Briefly, Caco2 cells were co-cultured with PMN’s as described above. Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers. After 24 hours, cells were washed twice with cold PBS, then scraped from dishes. Protein concentrations were determined using Bradford assays (28). 30 mg protein was dissolved in an SDS-PAGE sample-loading buffer, electrophoresed in 10% Tris-Glycine pre-cast gels, and transferred onto nitrocellulose sheets. After blocking in 5% milk in TTBS (0.01 M Tris/0.15 M NaCl buffer, pH 8.0 and 0.1% Tween 20) for one hour at room temperature, blots were incubated with the primary antibody (rabbit polyclonal anti-NOS2 lgG, 1:100) overnight at 4oC. Blots were then incubated with goat-anti-rabbit IgG (1:4000) for one hour at room temperature, washed, and treated with ECL Western blotting detection solution. Subsequently, blots were exposed to film, films were developed and digitally scanned, and iNOS expression was analyzed via densiometric analysis (Scion Image for Windows, beta 4.0.2, Scion Image Co., Frederick, MD).
Concentrations of TNF, IL-1β, and IL-6 present in basolateral media were determined via commercially available ELISA kits (BD Biosciences) using an “Emax” microplate reader (Molecular Devices, Sunnyvale, CA).
Data Analysis
Data were analyzed using SPSS software (SPSS 11.0.1, Chicago, IL). Statistical analyses were conducted using ANOVA; Tukey’s HSD tests were used for post-hoc evaluations. P<0.05 was considered significant. Data are presented as mean ± standard deviation.
Results
fMLP Transport
Cephalexin inhibited fMLP movement across the Caco2 monolayer, presumably via inhibition of hPEPT1 mediated permeation of fMLP. Specifically, cephalexin 10 mM significantly decreased the Peff of fMLP, however this was not seen with cephalexin 5 mM (p=0.007, figure 2). Based on these results, cephalexin 10 mM was used for subsequent experiments.
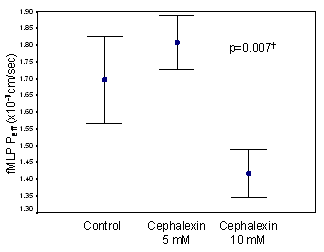
Figure 2. fMLP transport across Caco2 monolayers. 3H fMLP (0.1 mM) was added to the apical chambers of permeable supports, with and without cephalexin 10 mM. Basolateral concentrations of 3H fMLP were sampled at 0, 15, 30, 45, 60, and 120 minutes and Peff was determined as described in the text. n=3 wells/group.
†p<0.05 for “cephalexin10 mM” vs. “control” and “cephalexin 10 mM” vs. “cephalexin 5 mM”.
fMLP-Induced Intestinal Hyperpermeability
The effects of fMLP induced PMN migration on Caco2 monolayer integrity were evaluated in the PMN co-culture model by measuring mannitol Peff and TEER. In the presence of fMLP and inflammatory cytokines, mannitol permeability increased and TEER decreased, indicating monolayer hyperpermeability (figure 3. and figure 4.). Of interest, this increase in monolayer permeability was only evident in the presence of inflammatory cytokines. Cephalexin 10 mM almost completely attenuated changes in mannitol permeability (figure 3, p<0.05), although it had minimal effects on TEER (figure 4).
iNOS Expression and Cytokine Production
iNOS-mediated NO production is a key event in cytokine induced hyperpermeability of cultured intestinal monolayers. In order to determine whether cephalexin associated changes in fMLP transport resulted in alterations in intestinal markers of inflammation, iNOS expression in Caco2 cells was determined following co-culture with PMN’s, in the prese nce and absence of fMLP and cephalexin. As illustrated in figure 5, iNOS expression was highly variable and was not affected by cephalexin. Similar results were obtained when iNOS expression was corrected for the expression of the constitutively expressed intestinal protein villin (data not shown). Concentrations of the inflammatory cytokines TNF, IL-1β, and IL-6 in the basolateral media were also determined. Cephelaxin attenuated TNF production in the co-culture system (in the presence of fMLP) compared to monolayers co-cultured with PMN’s/fMLP without cephalexin (figure 6a.). In contrast, concentrations of the inflammatory cytokines IL-1β, and IL-6 were unaffected by cephalexin (figures 6b and 6c.)..
Discussion
Mucosal hyperpermeability plays a major role in the pathogenesis and sequelae of a number of inflammatory disease states, including sepsis, burn injury, inflammatory bowel diseases, and others. To this end, a number of novel strategies have been studied in models of injury-induced intestinal changes; most of these strategies involve the use of compounds with anti-inflammatory, antioxidant, and/or trophic effects at the level of the intestinal mucosal (29-32). Unfortunately, most of these strategies have not been employed clinically, and controversy exists regarding the clinical utility (i.e., in preventing intestinal dysfunction) of those strategies that have been used in the clinical arena. In this study, we investigated the potential to modulate intestinal dysfunction via inhibition of the uptake of the N-formylated chemotactic peptide fMLP by an intestinal peptide transporter, using the peptidomemitic drug cephalexin.
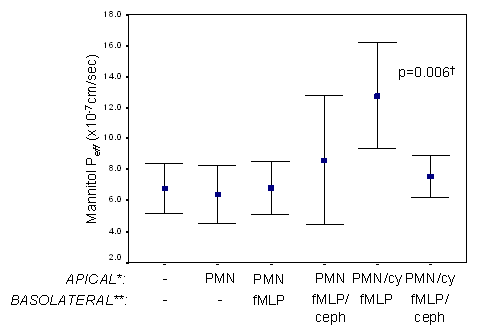
Figure 3. Effects of cephalexin on mannitol Peff in Caco2 monolayers co-cultured with polymorphonuclear leukocytes. Polymorphonuclear leukocytes (7 x 106 cells/ml) were added to apical chambers of permeable supports in the presence and absence of a mix of inflammatory cytokines (IFN-γ, IL-1β, TNF). Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers. 3H mannitol Peff was determined after two hours as described in “Methods” section. n=5 wells/group. *cy=cytokine mix. PMN= polymorphonuclear leukocytes. **ceph=cephalexin. †p<0.05 for polymorphonuclear leukocytes /cytokine--fMLP” vs.: “control”, “polymorphonuclear leukocytes”, “polymorphonuclear leukocytes--fMLP”, “polymorphonuclear leukocytes/cytokine--fMLP/cephalexin”.
N-formylated peptides are believed to play an integral role in mediating disease-induced alterations in intestinal permeability. fMLP, the “prototypical” N-formylated peptide, causes intestinal hyperpermeability via a number of mechanisms, including stimulation of leukocyte chemotaxis, activation of monocytes and neutrophils, and activation of the 5-lipoxygenase pathway (13,16,17,19). Of these mechanisms, polymorphonuclear leukocyte (PMN) migration across the intestinal cell wall is thought to be the most important cause of N-formylated chemotactic peptide induced intestinal dysfunction (i.e., during the migration process, PMN’s physically disrupt the tight junctions of the intestinal epithelia). (14,18,20).
The human oligopeptide transporter, hPEPT1, is a proton-coupled transporter present in the brush-border membrane of the small intestine that mediates the active intestinal absorption of di- and tri-peptides (33). In addition to the transport of oligopeptides, this transporter is also responsible for the intestinal absorption of a number of peptidomimetic pharmaceuticals, including β-lactam antibiotics, angiotensin converting enzyme inhibitors, antivirals, antineoplastics, and others, and has been targeted as a means of increasing the bioavailability of poorly absorbed drugs (27,34). Of interest, hPEPT1 appears to be upregulated in response to physiological stress. We have demonstrated that the inflammatory cytokine IFN-γ increases hPEPT1 expression and hPEPT1 mediated transport of the model dipeptide glycylsarcosine (Gly-Sar) in cultured human intestinal monolayers (35). Others have confirmed that this increase in hPEPT1 expression results in an increase in hPEPT1 mediated fMLP absorption (36). Further, although hPEPT1 is not normally expressed in the colon, colonic expression of hPEPT1 occurs in inflammatory bowel disease (perhaps explaining why N-formylated peptides do not affect intestinal integrity under normal conditions, however do so in pathologic conditions) (25).
Other investigators have demonstrated that the model dipeptide Gly-Sar inhibits fMLP uptake and PMN migration in Caco2 cells and in perfused rat jejunum (37,38). To the best of our knowledge, the current study represents the first investigation of a pharmacologic hPEPT1 substrate (i.e., cephalexin) as a means of decreasing intestinal fMLP transport and PMN migration. In the present study, we demonstrated that cephalexin decreases absorption of fMLP, presumably via inhibition of hPEPT1 mediated fMLP uptake (figure 2). Moreover, in a co-culture model with human PMN’s, cephalexin attenuated fMLP mediated intestinal dysfunction, as indicated by a reduction in the Peff of the marker mannitol (figure 3). Of interest, the observed reduction in membrane electrical resistance (TEER, figure 4) was not attenuated, as has been the case in other investigations (18). This may relate to several factors. First, our laboratory and others have reported cytokine-induced reductions in TEER, so the observed changes in TEER may be partially attributed to the inflammatory cytokines, which we would not expect to be attenuated by cephalexin (35,39). Moreover, TEER is a relatively nonspecific measure of changes in permeability, and alterations in TEER may not correspond to alterations in macromacule permeability. In our PMN migration studies, we used a mixture of inflammatory cytokines (TNF, IL-1β, and IFN-γ) in order to “prime” PMN’s. Our rationale for this was two-fold; first, cytokine priming increases PMN responsiveness to N-formylated peptides, and second, this may better mimic in vivo states of inflammation (40). We did not observe significant changes in intestinal permeability with PMN’s in the absence of inflammatory cytokines, whereas other studies have observed such changes (18,41). Although we are unable to fully reconcile these differences, they may relate, in part, to the use of different assessments of permeability (i.e., choice of marker).
Induction of iNOS expression is a key event in cytokine induced intestinal hyperpermeability, and attenuation of iNOS expression is a commonly employed surrogate when evaluating strategies to prevent intestinal dysfunction (12). Therefore we evaluated iNOS expression in order to determine if iNOS plays a role in PMN mediated changes in intestinal dysfunction, and if so, whether cephalexin mediated changes in fMLP transport influence iNOS expression. iNOS expression was not altered in this model, suggesting that PMN mediated intestinal dysfunction may not be related to iNOS induction (figure 5.). The co-culture of PMN’s with Caco2 monolayers resulted in the production of TNF, IL-1β, and IL-6 (presumably a response mainly mediated by PMN’s, although it was not possible to differentiate between PMN and Caco2 cytokine production) (figure 6.). In the case of IL-1b and TNF, this production increased in the presence of fMLP (figures 6a. and 6b.). Notably, TNF production was partially attenuated by cephalexin, potentially suggesting that inhibition of fMLP uptake may prevent PMN mediated TNF production (figure 6a).
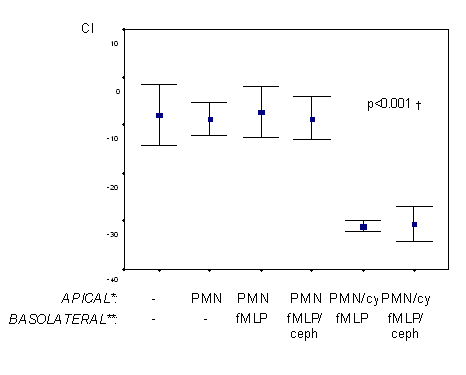
Figure 4. Effects of cephalexin on transepithelial resistance (TEER) in Caco2 monolayers co-cultured with polymorphonuclear leukocytes. Polymorphonuclear leukocytes (7 x 106 cells/ml) were added to apical chambers of permeable supports in the presence and absence of a mix of inflammatory cytokines (IFN-γ, IL-1β, TNF). Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers. Change in TEER was determined after two hours. n=5 wells/group. *PMN= polymorphonuclear leukocytes; cy=cytokine mix. **ceph= cephalexin. †p<0.05 for “polymorphonuclear leukocytes/cytokine--fMLP” and “polymorphonuclear leukocytes /cytokine--fMLP/cephalexin” vs. other groups.

Figure 5. Expression of iNOS in Caco2 monolayers co-cultured with polymorphonuclear leukocytes. Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers. After 24 hours, iNOS expression was determined by Western blotting as described in the “Methods” section. A representative blot is shown in figure 5a., and the results of the densiometric analysis (n=3 wells/group) are illustrated in figure 5b. * PMN= polymorphonuclear leukocytes. **ceph=cephalexin. Our study may be associated with several limitations. First, during PMN migration experiments, it would be more physiologically relevant to place PMN’s in the basolateral chamber (i.e., as this chamber corresponds to the serosal side of the Caco2 cells). Although this is a theoretical limitation, others have demonstrated that fMLP-stimulated PMN migration in the apical to basolateral direction is similar to that in the basolateral to apical direction, and that this experimental design may be used to study PMN related intestinal dysfunction (18,42). In our PMN co-culture experiments, the pH of the media was not optimized to facilitate hPEPT1 activity as it was in fMLP transport experiments; rather, in the co-culture experiments, media suitable for PMN function was used. This strategy, however, has been used successfully in other investigations (19,38).

Figure 6. Effects of cephalexin on cytokine production in Caco2 monolayers co-cultured with polymorphonuclear leukocytes. Caco2 cells were co-cultured with polymorphonuclear leukocytes (as described in “Methods” section). Control media or media containing fMLP (1 µM) with and without cephalexin (10 mM) was added to basolateral chambers. After 24 hours, concentrations of TNF (figure 6a.), IL-1β (figure 6b.), and IL-6 (figure 6c.) present in basolateral media were determined using ELISA’s. n=3 wells/group. n=3/group. * PMN= polymorphonuclear leukocytes. ** ceph=cephalexin.
†p<0.05 for “control” vs. “polymorphonuclear leukocytes --fMLP”. ‡p<0.05 for “control” vs. “polymorphonuclear leukocytes --fMLP” and “control” vs. “polymorphonuclear leukocytes --fMLP/ cephalexin”.
The concentration of cephalexin employed in our study (10 mM) is higher than the concentration that would be expected to be achieved systemically; cephalexin, however, is administered orally, meaning that relatively high concentrations are present at the site of interest for this study, the intestine. Of note, for some experiments, the number of samples studied was relatively small; beacus limited data were available upon which to base sample size calculations, sample sizes were selected based on previous experience with similar types of studies. We observed substantial variability in our markers of inflammation (iNOS expression and cytokine production). This was not necessarily unexpected; as certain amount of “cross talk” between the two cell types is anticipated.
In summary, cephalexin significantly reduced fMLP transport across cultured human intestinal monolayers. This resulted in an attenuation of intestinal dysfunction. Because iNOS expression and cytokine production were largely unaffected by cephalexin treatment, it is likely that the effects of cephalexin were primarily mediated by inhibition of fMLP-mediated PMN transmigration. These phenomena may have importance regarding the use of enteral peptidomimetic drugs and nutritional oligopeptides in patients with diseases related to intestinal hyperpermeability. Specifically, these data suggest that it may be possible to employ cephalexin (and other hPEPT1 substrates) to attenuate the detrimental effects of fMLP. Manipulation of the intestinal bacterial flora with antimicrobials and probiotic agents has been tried clinically with mixed results in the critically ill and in patients with inflammatory bowel disease (6,43). Given our current results, a rational extension of this research may be to study the use of antimicrobial agents that are also hPEPT1 substrates as part of such strategies. Ultimately, future studies evaluating potential clinical applications of these results are warranted.