J Pharm Pharmaceut Sci (www.cspscanada.org) 10(1):1-8, 2007
Anesthetic Effects of Lidocaine Hydrochloride Gel using Low Frequency Ultrasound of 0.5 MHz
Tae-Youl Kim, Dae-In Jung, Young-Il Kim, Jae-Heon Yang, and Sang-Chul Shin
Department of Physical Therapy, Dongshin University, Naju 520-714, Korea Department of Pharmaceutical Engineering, Konyang University, Nonsan, 320-711, Korea Department of Pharmacy, Woosuk University, Samrye 565-701, Korea College of Pharmacy, Chonnam National University, Gwangju 500-757, Korea
Received, November 11, 2006; Revised, January February 5, 2007; Accepted, February 10, 2006; Published, February 15, 2007.
Corresponding Author: Prof. Sang-Chul Shin College of Pharmacy Chonnam National University 300 Yongbongdong, Buggu Gwangju 500-757 South Korea Tel:+82-62-530-2924 Fax:+82-62-530-2949 E-mail: shinsc@chonnam.ac.kr
ABSTRACT -- Purpose. Low-frequency ultrasound has a significant effect on the transdermal permeation of high molecular weight drugs. However, the rate of permeation in pulsed mode is quite low necessitating considerable time to apply the ultrasound. 0.5 MHz ultrasound, which is a relatively higher frequency in the low-frequency range, can be applied in high intensity in continuous mode. Methods. A transducer was used to administer an anesthetic drug transdermally on healthy volunteers. The anesthetic effect was measured following administration on placebo, lidocaine HCl alone and lidocaine HCl with 0.5 and 1.0 MHz ultrasound (n=8/group). Results. In surface anesthesia, the phonophoresis group showed a significantly higher pain threshold than the other groups but there was no significant difference between the phonophoresis groups according to the ultrasound frequency. In conduction anesthesia, the 0.5 MHz phonophoresis group showed a significant change in their pain threshold and amplitude of sensory nerve action potential (SNAP) compared with the other groups. Conclusions. Although there are limitations in applying 0.5 MHz ultrasound in phonophoresis for conduction anesthesia using lidocaine hydrochloride for a nerve block, it is more effective than the 1 Mhz that is widely used in clinical situations.Carbamazepine is a poor water soluble drug and its bioavailability is limited by dissolution rate. Dissolution, serum concentration and anticonvulsive effect of the drug have been evaluated after cogrinding with microcrystalline cellulose. A cogrinding technique was used to increase the dissolution, serum concentrations and anticonvulsive effect of the drug. A novel deconvolution technique of in vitro- in vivo correlation was evaluated.
Introduction
Very high frequency sound (< 20 kHz) is usually created when a generator produces electrical energy that is converted to mechanical energy through the deformation of the piezoelectric material in a transducer (1). The waves produced are transmitted by propagation through molecular oscillation, with a progressive loss of acoustic energy during passage through biological tissue due to the absorption or scattering of the sound waves (2). When the ultrasound interacts with tissues, it causes changes as a result of thermal (heating) and mechanical effects (pressure, cavitation and acoustic streaming) (3). Phonophoresis is used in various treatments by introducing ultrasound energy into the body (4). Phonophoresis is divided into low frequency phonophoresis (< 1 MHz), therapeutic frequency phonophoresis (between 1 MHz and 3 MHz) and high frequency phonophoresis (between 3 MHz and 15 MHz) depending on the ultrasound frequency (5). Most studies and clinical applications are within these ranges (6 - 8). In particular, therapeutic frequency phonophoresis has been used clinically to assist in the permeation of various drugs such as lidocaine (9), hydrocortisone (10), prednisolone (11), and triamcinolone acetonide (12). Therapeutic frequency ultrasound can enhance the transdermal permeation of low molecular weight drugs (3). However, the enhancing effect of transdermal permeation decreases with increasing molecular weight e.g. fluocinolone acetonide, benzydamine, nicotinate, salicylate, etc. (13 - 15). On the other hand, it has been reported that low frequency ultrasound of 0.02 ~ 0.2 MHz generates significant energy and allows the deep transdermal permeation of drugs that are difficult to permeate at the therapeutic frequency (16, 17).
A special method is needed for transdermal permeation due to the semi-solid properties of lidocaine (18). Therefore, many studies have focused on enhancing the level of transdermal permeation by either changing the physiochemical properties of lidocaine or using physical modalities (19 – 23). In particular, there has been considerable interest in transdermal permeation using various methods based on the idea that the amount and depth transdermal permeation can be increased using various physical modalities. However, it is difficult to expect more than anesthetic effects (24). Becker et al. (25) reported that phonophoresis deepened the depth of penetration of drugs compared with iontophoresis, and that the depth of penetration by ultrasound varied according to the frequency. The absorption of ultrasound energy in the tissue decreases with decreasing frequency (26). If the frequency is reduced, the amount of energy absorption in the superficial tissue is low, and the ultrasound can reach the required depth. Therefore, the use of low frequency ultrasound is very efficient in helping a drug to reach the appropriate depth, increasing the temperature of the deep tissue efficiently and enhancing the transdermal permeation of drugs (27). The ultrasound frequencies used in the phonophoresis of lidocaine have been 1.1 MHz (27) and 0.87 MHz (13). Studies examining low frequency ultrasound have used 0.048 MHz (28) and 0.053~0.056 MHz (25). While most studies suggest that the surface anesthetic effects are great, the deep anesthetic effects according to the increase in the depth of penetration of a drug were not so great. Therefore, this study examined the deep anesthetic effects of ultrasound using a 0.5 MHz ultrasound apparatus, which is a relatively high frequency in the low frequency region ultrasound, and an ultrasound transducer.
Materials and METHODS
Chemicals and Reagents
Lidocaine hydrochloride was gifted from Daihan Pharm. Co. Ltd (South Korea). Carbomer 940 and Transcutol® were from Gattefossé (France). Triethanolamine, propylene glycol, methyl paraben, propyl paraben, and epinephrine were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the reagents were of analytical grade and used without further purification.
Gel preparation
A 1 % (w/w) lidocaine hydrochloride (HCL) gel was prepared. Briefly, 1.0g of carbomer 940 was dissolved in 13.7 mL of water. Triethanolamine was then added with constant agitation to make a gel with a pH 6.5 ~ 7.5. 1.0g of lidocaine was dissolved in 10 mL of Transcutol® and 20 mL of propylene glycol. The above mixture was combined, and water was added to make 100 mL. The gel for the clinical experiment contained methyl paraben, propyl paraben (0.02: 0.03%) and epinephrine of 1/100,000 as preservatives.
Phonophoresis
A 0.5 MHz ultrasound apparatus (USTD-K, Daeyang Medical Co., Korea) was manufactured for this experiment. According to technical specifications of the ultrasound apparatus, the frequency was 0.5 MHz, the pulse duration was 200ms, the duty cycle was 20%, the peak to peak amplitude was 600 mV and the output intensity was 0~5 Watts. The 1 MHz ultrasound apparatus used was a USTD-M (Daeyang Medical Co., Korea) with a 1 MHz frequency, and an output of 0~5 Watts. The ultrasound was applied to the right wrist where the median nerve runs. The treatment dose of the blank group was set to 2.0 W/cm2 SATAi (spatial average temporal average intensity) after applying a commercial ultrasound gel. The 1 MHz and 0.5 MHz phonophoresis groups received the same ultrasound energy as the blank group after using 5 g of the 1 % lidocaine hydrochloride gel.
Subjects
The ethical committee at the University of Konyang approved this study and all procedures were in accordance with the ethical guidelines. Thirty-two healthy male volunteers, who provided informed consent, were enrolled in this study. Those with diseases influencing the experiment such as sensory impairment, pain due to an injury to the hand and those who were taking medications for these symptoms were excluded. The subjects were prohibited from using alcohol or drugs prior to the study. They were randomly classified into 4 groups (8/group). The blank group received a 0.5 MHz ultrasound treatment after applying the ultrasound gel without lidocaine hydrochloride. The group that did not receive the ultrasound treatment but only the lidocaine HCl gel was classified as the lidocaine HCl group. The 1 MHz phonophoresis group received a 1 MHz ultrasound treatment after applying the lidocaine HCL gel. The 0.5 MHz phonophoresis group received 0.5 MHz ultrasound treatment after applying the lidocaine HCl gel.
Quantitative sensory testing (QST)
The threshold was measured at two places. After applying the gel and ultrasound, the surface anesthetic effects were measured at the volar surface of the right wrist including the tract of the median nerve, and the nerve block effect by conduction anesthesia was measured at the volar surface of the right index finger (29). The pressure pain threshold was measured before and after 30, 60 min of treatment. Von Frey monofilaments (Touch-Test Sensory Evaluation, North Coast Medical Inc., USA), with a pressure range of 10, 15, 26, 60, 100, 180 and 300 g, were used to measure the pressure pain threshold (PPT) (30). The Von Frey monofilament was kept perpendicular to the skin, and pressure was applied until the microfilament bent. The average values were taken by repeating the initial gram recognizing pressure pain three times (31). A halogen lamp (Osram Co., Germany) and a digital thermometer (CENTER-306, Center Technology Co., Taiwan) were used to measure the thermal pain threshold (TPT). A thermometer was connected to a PC with a thermolog computer linking program with the temperature being recorded every 1second. The thermal pain threshold was measured at the same region used to measure the pressure pain threshold (29, 30). The tip of the wire sensor was fixed with adhesive tape to the center of the measurement site and was adjusted to be perpendicular to the light source. A distance of 5cm was maintained. The temperature at which pain was first noticed (32) was used to measure the TPT
Nerve conduction study (NCS)
The nerve conduction study (33) was carried out at the median nerve using diagnostic EMG (Sierra II wedge, Cadwell laboratories Inc., USA). The sensory nerve action potential (SNAP) was measured using the antidromic method. The sweep was set to 2 ms/division and the gain was set to 20 μV/division. The recording electrode was placed on the right index finger using a ring electrode (Elect. Digital Ring 24, Cadwell laboratories Inc., USA) and the distance between the electrode and the electrical stimulation site was fixed at 14 cm. The record conditions of the motor nerve action potential (MNAP) included a sweep of 10 ms/division and a gain of 1 mV/division. The recording electrode was placed at the abductor pollicis brevis using a disposable EMG electrode (Ambu® Neuroline 700, Ambu, Denmark), and the distance between the electrode and the electrical stimulation site was fixed at 8 cm. The ground electrode was positioned at the dorsal surface of the hand. Electrical stimulation was applied between the palmaris longus, where the median nerve tract was positioned above the wrist joint and the tendon of flexor carpi radialis. Each action potential was measured three times and the average value was used for further analysis.
Data analysis
Statistical analysis was performed using the SPSS 10.0 version for windows program. All the values are reported as a mean ± standard error (S.E.). The difference between the groups according to the measuring periods was examined using a one-way ANOVA and a Tukey post hoc test. The significance level in the analysis was set to α=0.05.
RESULTS
Effect on the surface anesthesia
The PPT of the wrist region in the phonophoresis groups, which was measured to determine the effect of surface anesthesia, showed an increasing trend in the PPT. One-way ANOVA revealed a significant difference between the groups after 30 min application (P<0.01), and the post hoc test showed a difference between the phonophoresis groups and the blank group (Fig. 1). The TPT increased significantly after 30 min (P<0.01) and 60 min application (P<0.001), and the post hoc revealed a difference between the blank group and the phonophoresis group or lidocaine HCL group (Fig. 2).
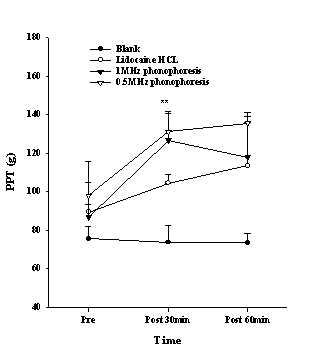
Figure 1. The pressure pain threshold (PPT) in the wrist after various treatments. Data points are mean±S.E. Significant differences were found between the phonophoresis groups and the blank group at 30 min; **P <0.01
Effect on the conduction anesthesia
The PPT of the index finger, which was measured to determine the effect of conduction anesthesia, showed an increasing trend in the 0.5 MHz phonophoresis group. There was a significant difference after 30 min application (P<0.001), and the post hoc showed a difference between the 0.5-MHz phonophoresis group and the blank group, lidocaine HCL group and 1 MHz phonophoresis group (Fig. 3). The TPT showed a significant difference after 30 min (P<0.05), and 60 min application (P<0.01), and the post hoc indicated a difference between the 0.5-MHz phonophoresis group and the blank group (Fig. 4). The amplitude of the SNAP showed a significant difference after 30 min (P<0.01), and 60 min application (P<0.01). The post hoc showed a difference between the 0.5 MHz phonophoresis group and the blank and lidocaine HCL groups (Fig. 5).
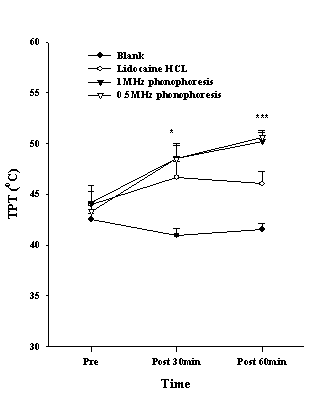
Figure 2. The thermal pain threshold (TPT) in the wrist after various treatments. Data are presented as the mean±S.E. Significant differences were found between the blank and the phonophoresis as well as the lidocaine HCL group at 30 and 60 min; **P <0.01, ***P <0.001
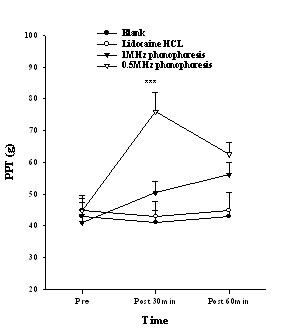
Figure 3. The pressure pain threshold (PPT) in the index finger after various treatments. Data are presented as the mean±S.E. At 30min the 0.5 MHz phonophoresis group was significantly different from other groups (***P <0.001).
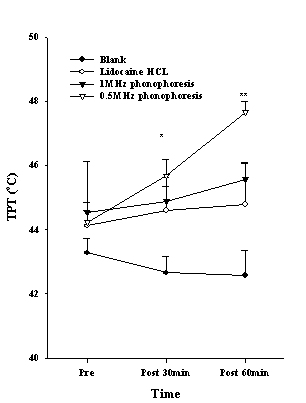
Figure 4. The thermal pain threshold (TPT) in the index finger after various treatments. The data points are mean±S.E. Significant differences were found between the 0.5 MHz phonophoresis and the blank group at 30 and 60 min (*P <0.05, **P <0.01).
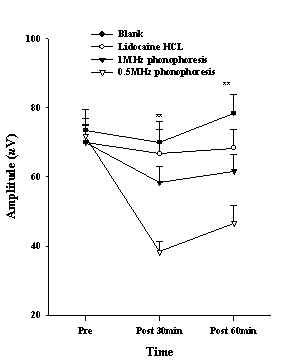
Figure 5. The sensory nerve action potential of the amplitude in the median nerve after various treatments. Data are presented as the mean±S.E. Significant differences were found between the 0.5 MHz phonophoresis group and the blank group, lidocaine HCL group at 30 and 60 min; **P <0.01
discussion
Topical lidocaine hydrochloride has been marketed in many applications such as a spray, lotion and cream, and has been used clinically to mitigate skin disease, light operations and local pain. However, its use has been limited to surface anesthesia because a conduction anesthetic effect with deep transmission is almost impossible. Various methods for improving the level of transdermal permeation and the permeation depth of drugs using various physical modalities have been examined. Deep anesthesia and conduction anesthesia by lidocaine hydrochloride requires an injection (34). However, the application of a lidocaine hydrochloride injection can cause pain and tissue damage, and its application in children is not recommended (35). Transdermal preparations in various forms have been manufactured to avoid these problems but it is difficult to expect effects other than surface anesthesia (21). Phonophoresis using low frequency ultrasound has been found to be relatively effective. The enhancement of transdermal permeation of lidocaine hydrochloride using phonophoresis is effective in surface anesthesia (20), but not so in deep anesthesia. On the other hand, when ultrasound frequency is low, the absorption of ultrasound energy by the superficial tissue decreases, the half-value thickness increases and the level of permeation can be deeper (36). Becker et al. (25) verified the deep anesthesia in a phonophoresis experiment with lidocaine cream using 0.053~ 0.056 MHz ultrasound. The results in the present study indicate significant changes in the PPT and TPT in the wrist after applying a lidocaine HCL gel with either 0.5 MHz or 1 MHz phonophoresis for 30 min (Figs. 1, 2). Friedman et al. (37), Katz et al. (38) and Becker et al. (25) reported that the superficial pain threshold was increased using 55 kHz ultrasound, a eutectic mixture of local anesthetics (EMLA) cream or liposomal lidocaine. Tang et al. (39) and Barcohana et al. (40) reported that the heat-induced pain threshold and PPT was increased using an EMLA cream. However, there was no change in the pain threshold in the blank group. It is assumed that the physiological effects of ultrasound increase the rate of cell membrane permeability and diffusion, increases the intracellular calcium level, and changes the electrical activity of the nervous tissue (41, 42). Therefore, it does not have a large influence in increasing the pain threshold.
The conduction block effects of lidocaine hydrochloride were examined by determining the PPT and TPT, and carrying out a nerve conduction study at the index finger, which is the area of the median nerve. There was a significant increase in the altered PPT between the 0.5 MHz phonophoresis group and the other groups after 30 and 60 minutes of application (Fig. 3). Therefore, phonophoresis using 0.5 MHz ultrasound is quite effective in blocking the mechanical pain compared with applying 1 MHz ultrasound or lidocaine hydrochloride only. Similarly, the influence of on the TPT was significantly greater in the 0.5 MHz phonophoresis group compared with in the other groups (Fig. 4), and was effective in blocking thermal pain to some degree. However, it was inferior in blocking mechanical pain. This suggests that when ultrasound passes through the human body, the half-value thickness increases with decreasing frequency (43), which in turn increases the depth of permeation as well as and the pressure wave caused by the increase in acoustic streaming. On the other hand, the blocking effect was reduced because the arrival of drugs at the deep tissue was prevented by the diffusion or delivery of the drug to the bloodstream, which is unlike that observed in in-vitro experiments (44). The amplitude of SNAP was different between the 0.5 MHz phonophoresis group and other groups (Fig. 5). Therefore, an appropriate permeation depth and volume will be needed if lidocaine hydrochloride is to affect the nerve conduction velocity. Moreover, because ultrasound causes biological changes within the tissue through thermal and mechanical effects, it can activate the transcellular or intercellular routes and enhance the level of transdermal permeation (45, 46). Hence, the ultrasound frequency and intensity can be important variables in determining the level of transdermal permeation and the depth of the drugs. When a nerve is blocked by local anesthesia, a delay in the latency of the sensory and motor nerves and a decrease in the amplitude can occur (47).
When the ultrasound energy enters to the body, it can interact with tissues and affect the cells and tissues through thermal (heating) and non-thermal mechanisms (pressure, cavitation and acoustic streaming) (3, 48, 49). Therefore, the ultrasound can be used to treat inflammation and help repair wounds by relaxing the soft tissue, and increasing the blood flow due to its thermal effects (50). The ultrasound energy itself can change the nerve conduction velocity slightly but has no effect on the conduction block (51). However, the thermal and mechanical effects of ultrasound can accelerate the deep permeation of local anesthesia such as lidocaine. Therefore, the effect of a conduction block in nerve trunk could be expected. The lidocaine hydrochloride content is also an important variable for determining the level of anesthesia. The lidocaine content hydrochloride used for transdermal application was varied from 1% to 10% (52, 53, 29), but no acceptable level of conduction anesthesia could be achieved using this transdermal application, and an injection is most reliable when a block of deep pain or conduction anesthesia by a nerve block are needed. Generally, 1 ~ 2% lidocaine is used when injected, (53, 54). Ririe et al. (29), who used a conducting nerve block with 1% lidocaine hydrochloride, reported that there changes in the sensation of warmth, coldness, mechanical sense, compound motor nerve action potential and sensory nerve action potential. Hence, 1% lidocaine was used in this study because the permeation depth of 0.5 MHz ultrasound was remarkably higher than with 1 MHz.
CONCLUSION
In surface anesthesia, the phonophoresis group showed a significantly higher pain threshold than the other groups. In conduction anesthesia, the 0.5 MHz phonophoresis group showed a significant change in their pain threshold and amplitude of sensory nerve action potential (SNAP) compared with the other groups. In addition, it was found that deep permeation of lidocaine improved the anesthetic effect. Although there are limitations in applying 0.5 MHz ultrasound in phonophoresis for conduction anesthesia using lidocaine hydrochloride for a nerve block, it is more effective than the 1 MHz that is widely used in clinical situations.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (the Center for Healthcare Technology Development, Chonbuk National University, Jeonju 561-756, Republic of Korea).