Dotchi Exerowa
First of all, I would like to say, that I find the idea of creating “research standards” for asphaltenes and other colloid forming materials in petroleum, a very good one. Standardizing is a step forward in the process of characterization of petroleum components and their properties. It provides for more reliable quantitative interpretation of results and avoids ambiguity.
There are some parameters that have been identified through the study of thin liquid films (foam, emulsion or wetting films) and I believe they can also be applied to asphaltenes and other amphiphile substances in petroleum. These parameters define the thermodynamic and kinetic properties of thin liquid films.
Out of the pool of these characteristics I can suggest the critical concentration of bilayer formation, Cc. This parameter is related to the formation and stability of thin liquid films, ergo to amphiphile bilayers as well. They are models used to study the bilayer contact in disperse systems, i.e. the last stage of interaction between two phases. The microscopic theory of nanoscopical holes nucleation (see “Nucleation Mechanism of Rupture of Newtonian Black films.I. Theory”, J. Colloid Interface Sci., 77 (1980) 501-511, D. Kashchiev, D. Exerowa, and “Foam and Foam Films”, D.Exerowa, P.M.Kruglyakov, Elsevier Science, Amsterdam, 1998) presents a possibility to consider formation and stability of amphiphile bilayers (Newtonian black films, obtained from low molecular surfactants) from a uniform point of view.
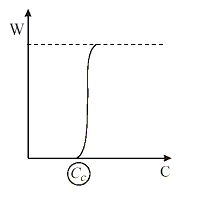
According to this theory, the dependence of the bilayer formation probability W on surfactant concentration Cs is very steep as shown in the figure. This allows not only to define the critical concentration of the bilayer formation but also to determine it very precisely. The measurement of Cc is a “yes/no” type of experiment, i.e. there is/there isn’t a bilayer. The microinterferometric apparatus of Scheludko-Exerowa would be the appropriate device to observe visually the bilayer – it looks black to the white light of the microscope (or video camera) – and to measure it. In fact the size of the microscopic film is of about 100 mm radius. This small film size allows determining the lowest concentration at which the black film is formed. According to the theory:
Cc = Ce exp [- B / ln ( tc / A)]
where tc = t (Cc), Ce - equilibrium concentration at which a thermodynamically stable film is formed; B = p A0 g2 / 2 (kT)2 , A0 is the area per molecule; g [J m-1] is the specific hole linear energy, and A [s] is a constant accounting for the nucleation kinetics.
Another approach to determining Cc would be describing amphiphile bilayers from polymer surfactants by the brush-to-brush interaction stabilizing them, which is applying scaling de Gennes’ theory. Here again W(C) dependence is measured but the course of the curve is determined by the change in the chemical potential.
Experimental results on Cc indicate that is a very sensitive parameter that depends on the surfactant’s type, its molecular mass, adsorption at the interface, etc., i.e. it is possible to create a “research standard” for asphaltenes and other colloid forming materials in petroleum.
Using the approaches mentioned it is possible to measure Cc in symmetric thin liquid films – foam and emulsion W/O/W films (using different solvents) and to study the bilayer formation.
In conclusion, I am convinced that the critical concentration of bilayer formation Cc can be introduced as a “research standard” for asphaltenes and other colloid forming materials in petroleum”. The reasons, in brief, are that this parameter:
- is very sensitive to the surfactant type (including polymer surfactant);
- is easy to measure; and
- can be measured precisely.
Should my suggestion be accepted we could consider a simple version of Scheludko-Exerowa microinterferometric apparatus for visual observation of the bilayers formed and run the experiments. Other types of materials such as resins, and other amphiphile molecules can also be considered for “standardization”.
Dotchi Exerowa, Professor, PhD, D.Sc.,
Head, Department of Colloid and Interface Science,
Institute of Physical Chemistry,
Bulgarian Academy of
Sciences